Abstract
Solvent-free copper(I) and thallium(I) compounds of the general formula [M2[B10H10]] have been synthesized. The compounds have been identified by IR spectroscopy and X-ray diffraction. According to X-ray diffraction data, the structures of both compounds are three-dimensional frameworks. In copper(I) complex [Cu2[B10H10]]n, the formation of three-center M–H–B bonds is observed. In thallium(I) compound Tl2[B10H10], the bond between cations and anions is ionic, which is confirmed by IR spectroscopy data. The Hirschfeld surface analysis of the boron cluster anion was performed for both compounds to identify and analyze intramolecular interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Boron cluster anions [BnHn]2– (n = 10, 12) [1–5] form an independent area of boron chemistry, which combines coordination, inorganic, organic, and organoelement chemistry. Coordination compounds of this class can participate in reactions of the substitution of terminal hydrogen atoms with various functional groups forming of a large number of substituted derivatives [6–9]. In addition, boron clusters and their substituted derivatives act as ligands forming metal complexes [10–15].
Among the great number of complexes with boron cluster anions, solvent-free complexes [Mx[BnHn]] (M is a metal atom, x = 1 (for metals in the oxidation state +2) or 2 (for metals in the oxidation state +1), [BnHn]2– is a boron cluster anion or its substituted derivative) are very few in number. The first complex of this kind, copper(I) complex [Cu2[B10H10]]n with the decahydro-closo-decaborate anion was obtained as early as 1962 [16, 17]. This complex was prepared when copper(II) acetate reacted with salts of the closo-decaborate anion in water. Complex [Cu2[B10H10]]n is the first compound where the bond between the metal atom and the closo-borate anion is realized due to three-center two-electron CuHB interactions. We note that in 1976, the author of this work, William Nunn Lipscomb, was awarded the Nobel Prize in Chemistry “for his studies on the structure of boranes, illuminating problems of chemical bonding”.
Metal complexes with boron cluster anions which were isolated later confirmed that this method of binding between a metal atom and a boron cluster is predominant one in complexes with coordinated boron cluster anions.
A similar copper(I) complex with the closo-dodecaborate anion [B12H12]2– was obtained by heating copper(II) aquacomplex with the closo-dodecaborate anion [Cu(H2O)6][B12H12] [18]. Complex [Cu2[B12H12]]n has a three-dimensional framework structure. The same authors obtained solvent-free cobalt(II) and nickel(II) complexes [М[B12H12]]n (M = Co, Ni) [18]. It was found that the compounds are built of infinite chains M–[B12H12]–M. Thermal treatment of manganese(II) and iron(II) complexes [Mn(EtOH)6][B12H12] and [Fe(MeOH)6][B12H12] containing six alcohol molecules led to the formation of solvent-free compounds [M[B12H12]]n (M = Mn, Fe), which have different structures [18]. The iron(II) complex, like the above mentioned cobalt and nickel complexes, is built of infinite M–[B12H12]–M chains, while manganese complex [Mn[B12H12]]n has a layered structure formed by trinuclear manganese clusters Mn3, which are bonded to boron cluster anions.
The synthesis and properties of thallium(I) salts with the [В10Н10]2– and [B12H12]2– anions were reported [19]. The compounds were obtained by the reaction between the corresponding closo-borate acids and thallium(I) hydroxide TlOH. A comparative analysis of the IR spectra and physicochemical properties of the two compounds allowed the authors to suggest a different nature of binding in both compounds Tl2[B10H10] and Tl2[B12H12]. In Tl2[B12H12] compound, the bond between the Tl(I) atom and the closo-dodecaborate anion is practically ionic. In a similar compound with the closo-decaborate anion, there is a significant fraction of the covalent component in the Tl(I)–[B10H10]2– anion bond. The authors conclude that the Tl(I) atom is bound to the apical boron atoms in the boron cage, but the participation of hydrogen atoms in the interaction is not discussed.
Later, when (H3O)2[B12H12] reacted with carbonate Tl2CO3 compound Tl2[B12H12] was isolated and its structure was determined by single crystal X-ray diffraction [20, 21]. This compound is isostructural with M2[B12H12] (M = K+, Cs+, Rb+) [22, 23]. Thallium atoms are located in tetrahedral voids between four [B12H12]2– anions; twelve hydrogen atoms of four triangular faces BBB of the closo-dodecaborate anions form an almost perfect cuboctahedral coordination sphere around each thallium atom; the Tl–H distances are 2.956 Å.
Solvent-free complexes were also obtained for substituted derivatives of boron cluster anions. Nickel compound [Ni[B12Cl12]] was isolated by heating aquacomplex [Ni(H2O)6][B12Cl12] ⋅ 6H2O at >450°C [24]. It has been found that, as the temperature rises, the nickel aquacomplex loses crystallization and solvate water molecules to form solvent-free compound [Ni[B12Cl12]].
The synthesis of silver(I) complexes [Ag2[B10H10]] [25, 26] and [Ag2[B12H12]] [27] has been described. The compounds are formed when silver nitrate reacted with salts of boron cluster anions and precipitate from aqueous solutions with a yield close to quantitative. The complexes were identified by elemental analysis and IR spectroscopy. The structure of the compounds has not been studied by X-ray diffraction.
When a similar reaction was performed for the decachloro-closo-decaborate anion, silver complex [Ag2[B10Cl10]]n was obtained as a result of prolonged evaporation of the reaction solution in air [14]. X-ray diffraction analysis showed that the silver(I) atom coordinates three boron cluster anions in a chelate manner to form an AgCl6 octahedron with Ag–Cl distances equal to 2.706(1)–2.893(1) Å. Each boron cluster anion is coordinated by six metal atoms, four metal atoms coordinate one apical and one equatorial chlorine atoms, and two metal atoms coordinate two equatorial chlorine atoms.
For the dodecachloro-closo-dodecaborate anion, similar complex [Ag2[B12Cl12]]n was synthesized [28]. Each [B12Cl12]2– anion is coordinated by the silver atom via two chlorine atoms, which leads to a six-coordination environment of the silver atom with a distorted octahedral geometry. The Ag–Cl bond lengths (2.83–2.85 Å) in this complex are comparable with the bond lengths in AgCl (2.77 Å) and in various silver compounds with chlorinated carborane anions. In compounds [Ag2[B10Cl10]] and [Ag2[B12Cl12]], the silver atom is in an octahedral environment, and the boron cluster anion is bonded to six metal atoms, but the structures of the three-dimensional frameworks are different.
In this work, the structure of the well-known polymer complex [Cu2[B10H10]]n is refined and a similar thallium compound Tl2[B10H10] is synthesized, the structures of the compounds are compared, the coordination features of the boron cluster anion are studied, and the nature of the metal bonds with the boron cluster formed in both structures is discussed.
EXPERIMENTAL
Triethylammonium closo-decaborate (Et3NH)2[B10H10] was synthesized from decaborane-14 through the formation of 1,6-bis(triethylamine)decaborane according to the procedure described [29].
Sodium closo-decaborate was obtained by boiling (Et3NH)2[B10H10] in aqueous sodium hydroxide solution until triethylamine was completely removed.
Synthesis of [Cu2[B10H10]]n (I). A solution of Na2[B10H10] in water (10 mL) was added to an aqueous solution of copper(II) sulfate (20 mL). Fine crystalline precipitate was formed. Yield with respect to boron, 41%. The crystal suitable for X-ray diffraction study was selected directly from the reaction solution.
IR (NaCl, Nujol mull, cm–1): 2565, 2539, 2512, 2144.
For Cu2B10H10 anal. calcd. (%): H, 4.11; B, 44.08; Cu, 51.82. Found (%): H, 4.03; B, 43.79; Cu, 51.32.
Synthesis of Tl2[B10H10] (II). A solution of TlNO3 (2 mmol) in water was added to a solution of (Et3NH)2[B10H10] (1 mmol) in water (10 mL). Within a week, the formation of colorless crystals was observed. The crystals were filtered off and dried in air. Yield, 67%.
IR (NaCl, Nujol mull, cm–1): 2523, 2458.
For Tl2B10H10 anal. calcd. (%): H, 1.91; B, 20.52; Tl, 77.57. Found (%): H, 2.03; B, 20.44; Tl, 76.69.
Elemental analysis was performed on a CHNS-3 FA 1108 Elemental Analyzer automatic gas analyzer (Carlo Erba). The boron and metal content was determined by the ICP MS method on an iCAP 6300 Duo inductively coupled plasma atomic emission spectrometer. For analysis, the samples were dried to constant weight.
IR spectra of the reagents and products were recorded on an Infralum FT-02 FT-IR spectrophotometer (SPF AP Lumeks, Russia): suspension in Nujol mull (Aldrich); NaCl plates; measurement range, 4000–400 cm–1; resolution, 1 cm–1.
X-ray diffraction. Sets of diffraction reflections were obtained at the Center for Collective Use of the Kurnakov Institute RAS on a Bruker SMART APEX2 (for crystals I) and a Enfaf Nonius CAD4 (for II) automatic diffractometers. The structures were solved by the direct method with subsequent calculation of difference Fourier syntheses. All hydrogen atoms were refined according to the rider model with thermal parameters Uiso = 1.2Ueq (Uiso) of the corresponding non-hydrogen atom. All non-hydrogen atoms were refined in the anisotropic approximation.
The APEX2, SAINT, and SADABS programs [30] were used for data collecting and processing. The structures were solved and refined using the programs of the OLEX2 complex [31].
The main crystallographic data, experimental parameters, and structure refinement characteristics are given in Table 1. Crystallographic data were deposited with the Cambridge Crystallographic Data Center (CCDC nos. 2118147 and 2118148).
Analysis of the Hirschfeld surface. The Crystal Explorer 17.5 program [32] was used to analyze interactions in crystals. Donor-acceptor groups are visualized using standard (high) surface resolution, and dnorm surfaces are displayed on a fixed color scale from –0.640 (red) to 0.986 ae (blue).
RESULTS AND DISCUSSION
Synthesis and Structure of Complex [Cu2[B10H10]]n
Complex [Cu2[B10H10]]n was synthesized by the reaction between copper(II) sulfate and the closo-decaborate anion according to the reaction [16, 17]:
In this case, the yield of the compound is low, since part of the anion is spent on the redox reaction with copper(II) atoms, which leads to partial degradation of the boron cluster anion.
Note that salt Na2[B10H10] was used to obtain the target compound, since the use of closo-decaborate with a large organic cation, for example, (Et3NH)2[B10H10], can result in anionic complex {(Et3NH)Cu[B10H10]}n. Complexes {Cat[Cu[B10H10]]}n were studied in detail [26, 33].
In the IR spectrum of compound I, a strongly split band of stretching vibrations of ВН bonds ν(BH) is observed with maxima at 2565, 2539, 2512, and 2144 cm–1, which indicates the involvement of the boron cluster anion in metal coordination.
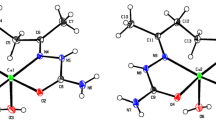
In contrast to data reported [16], the hydrogen atoms of the boron cluster in compound I are localized in the difference Fourier synthesis and refined independently in the isotropic approximation. The crystallographically independent part of the orthorhombic cell (space group Pbca) of complex [Cu2[B10H10]]n contains two copper atoms and one anion. A 2D polymer is formed in the crystal structure of the complex due to the formation of four pairs of three-center two-electron bonds CuHB of the boron cage with four copper atoms. Coordination is carried out along two opposite axial edges of one of the anion vertices (B1B2 and B1B4) and two adjacent edges of the other (B8B10 and B9B10). The Cu–B and Cu–H bond lengths are given in Table 2 and are consistent with the data reported for the complex [16].
The Cu–H contacts are shown in Fig. 1 (highlighted in color). Analysis of the Hirschfeld surface of the [B10H10]2– anion shows that H···H contacts with neighboring clusters account for 70.8% of the anion surface, H–Cu contacts account for 23.8% of the surface, and B–Cu contacts correspond to 5.4% (Fig. 2) .
Synthesis and Structure of Compound Tl2[B10H10]
Thallium(I) salt was obtained by reacting thallium nitrate with salt (Et3NH)2[B10H10] according to the reaction:
The compound is formed in high yield (67%).
The IR spectrum of the compound shows ν(BH) bands with maxima at 2523 and 2458 cm–1, which suggests that there is no coordination between the metal cation and the boron cluster anion, since the low-frequency band ν(BH)MHB of coordinated BH groups of the boron cluster is not found, the maximum of which is usually observed in the region of 2300–2200 cm–1.
Salt II is isostructural to the previously described salts of the closo-decaborate anion with alkali metals Na, K, Rb [34], similarly to salt Tl2[B12H12] [20, 21], which is isostructural to K, Rb, and Cs salts of the closo-dodecaborate anion [22, 23]. The crystallographically independent part of the monoclinic unit cell (space group P21/n) includes the [B10H10]2– anion and two cations that are in a common position (Fig. 3). The closo-decaborate anion is surrounded by eight Tl+ cations (Fig. 2) with distances Tl···B 3.17–3.65 Å (Fig. 2) and Tl···H 2.67–3.44 Å. As can be seen on the Hirschfeld surface of the boron cluster anion (Fig. 2a), the thallium cations are located above the centers of the faces of the closo-decaborate anion, and the lengths of the contacts Tl···B are less than the sum of their van der Waals radii (red color in Fig. 2b). In this case, the Tl···B contacts account for 1.9% of the anion surface, with the Tl–B distance less than the sum of their van der Waals radii. The Tl···H contacts account for 33.9%, and the remaining 64.2% are accounted for the H···H contacts of neighboring anions with the shortest distance of ~2.15 Å.
In each case, the thallium cation is surrounded by four faces of the boron cage (Fig. 3) of four different [B10H10]2– anions.
An analysis of the Hirschfeld surface of two compounds shows that the proportion of the M–B contact in the copper complex significantly increases, in contrast to salt II (5.4% compared to 1.9%), while the proportion of M–H contacts in complex I is 23.8%, and in salt II it is equal to 33.9%, which is related to the large ionic radius of Tl+.
The resulting complex compounds, the structure of which is a three-dimensional framework, can be used for the targeted synthesis of organometallic polymers (MOFs) [35–40] with the participation of boron cluster anions.
Note that complex compounds with boron cluster anions are used as extractants of heavy metal salts, components for obtaining thermal protective coatings, in medicine, catalysis, etc. [41–45].
CONCLUSIONS
Thus, we synthesized copper(I) and thallium(I) coordination compounds with the general composition [M2[B10H10]]. The compounds were studied by IR spectroscopy and single-crystal X-ray diffraction. In copper(I) complex [Cu2[B10H10]]n, the closo-decaborate anion is coordinated by metal atoms forming three-center M–H–B bonds; the Cu–B distances are 2.143(2)–2.320(2) Å. The IR spectrum of the compound contains the ν(BH)MHB band, which also indicates the coordination of the boron cluster anion. In thallium(I) salt Tl2[B10H10], it can be assumed that the binding of the cation to the anion is ionic; the Tl···B distances are 3.17–3.65 Å. The IR spectrum contains only ν(BH) bands of non-coordinated BH groups.
REFERENCES
N. N. Greenwood and A. Earnshaw, Chemistry of the Elements (Butterworth-Heinemann, 1997).
R. N. Grimes, Carboranes (Academic Press, London, 2016). https://doi.org/10.1016/B978-0-12-801894-1.09989-3
N. S. Hosmane, Boron Science: New Technologies and Applications (CRC Press, 2012).
Boron-Based Compounds: Potential and Emerging Applications in Medicine, Ed. by E. Hey-Hawkins and C. Viñas Teixidor (John. Wiley & Sons Ltd., 2018). https://doi.org/10.1002/9781119275602
I. B. Sivaev and V. I. Bregadze, Polyhedral Boron Hydrides in Use: Current Status and Perspectives (Nova Science Publishers, Hauppauge, 2009).
I. B. Sivaev, Russ. J. Inorg. Chem. 66, 1289 (2021). https://doi.org/10.1134/S0036023621090151
I. N. Klyukin, N. A. Selivanov, A. Y. Bykov, et al., Russ. J. Inorg. Chem. 65, 1547 (2020). https://doi.org/10.1134/S0036023620100113
A. V. Nelyubin, N. A. Selivanov, A. Y. Bykov, et al., Russ. J. Inorg. Chem. 65, 795 (2020). https://doi.org/10.1134/S0036023620060133
I. B. Sivaev, A. V. Prikaznov, and D. Naoufal, Collect. Czech. Chem. Commun. 75, 1149 (2010). https://doi.org/10.1135/cccc2010054
V. V. Avdeeva, E. A. Malinina, K. Y. Zhizhin, and N. T. Kuznetsov, Russ. J. Coord. Chem. 47, 519 (2021). https://doi.org/10.1134/S1070328421080017
E. A. Kravchenko, A. A. Gippius, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 65, 546 (2020). https://doi.org/10.1134/S0036023620040105
E. Y. Matveev, I. V. Novikov, A. S. Kubasov, et al., Russ. J. Inorg. Chem. 66, 187 (2021). https://doi.org/10.1134/S0036023621020121
E. A. Malinina, S. E. Korolenko, A. P. Zhdanov, et al., J. Cluster Sci. 32, 755 (2020). https://doi.org/10.1007/s10876-020-01840-5
V. V. Avdeeva, G. A. Buzanov, E. A. Malinina, et al., Crystals 10, 389 (2020). https://doi.org/10.3390/cryst10050389
S. E. Korolenko, V. V. Avdeeva, E. A. Malinina, et al., Russ. J. Coord. Chem. 46, 297 (2020). https://doi.org/10.1134/S1070328420050024
R. D. Dobrott and W. N. Lipscomb, J. Chem. Phys. 37, 1779 (1962). https://doi.org/10.1063/1.1733368
T. E. Paxton, M. F. Hawthorne, L. D. Brown, and W. N. Lipscomb, Inorg. Chem. 13, 2772 (1974).
E. Didelot, Y. Sadikin, Z. Łodzian, and R. Černýa, Solid State Sci. 90, 86 (2019). https://doi.org/10.1016/j.solidstatesciences.2019.02.005
O. A. Kanaeva, G. S. Klimchuk, and K. A. Solntsev, Russ. J. Inorg. Chem. 32, 803 (1987).
N.-D. Van, I. Tiritiris, and T. Schleid, Z. Anorg. Allg. Chem. 630, 1764 (2004). https://doi.org/10.1002/zaac.200470140
I. Tiritiris, N.-D. Van, and T. Schleid, Z. Anorg. Allg. Chem. 637, 682 (2011). https://doi.org/10.1002/zaac.201000457
I. Tiritiris, T. Schleid, K. Müller, and W. Preetz, Z. Anorg. Allg. Chem. 626, 323 (2000). https://doi.org/10.1002/(SICI)1521-3749(200002)626:2<323::AID-ZAAC323>3.0.CO;2-Q
I. Tiritiris and Th. Schleid, Z. Anorg. Allg. Chem. 629, 1390 (2003). https://doi.org/10.1002/zaac.200300098
F. M. Kleeberg, R. E. Dinnebier, and T. Schleid, Inorg. Chim. Acta 467, 147 (2017). https://doi.org/10.1016/j.ica.2017.07.066
E. L. Muetterties, J. H. Baltnis, Y. T. Chia, et al., Inorg. Chem. 3, 444 (1964). https://doi.org/10.1021/ic50013a030
E. A. Malinina, K. Yu. Zhizhin, I. N. Polyakova, et al., Russ. J. Inorg. Chem. 47, 1275 (2002).
V. V. Drozdova, E. A. Malinina, O. N. Belousova, et al., Russ. J. Inorg. Chem. 53, 1024 (2008). https://doi.org/10.1134/S0036023608070097
I. Tiritiris and T. Schleid, Z. Anorg. Allg. Chem. 629, 581 (2003). https://doi.org/10.1002/zaac.200390095
W. H. Knoth, H. C. Miller, J. C. Sauer, et al., Inorg. Chem. 3, 159 (1964). https://doi.org/10.1021/ic50012a002
G. M. Sheldrick, Acta Crystallogr., Sect. A 71, 3 (2015). https://doi.org/10.1107/S2053273314026370
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
P. R. Spackman, M. J. Turner, J. J. McKinnon, et al., J. Appl. Crystallogr. 54, 1006 (2021). https://doi.org/10.1107/S1600576721002910
E. A. Malinina, V. V. Drozdova, I. N. Polyakova, and N. T. Kuznetsov, Russ. J. Inorg. Chem. 53, 197 (2008). https://doi.org/10.1007/s11502-008-2008-y
K. Hofman and B. Albert, Z. Kristallogr. 220, 142 (2005). https://doi.org/10.1524/zkri.220.2.142.59144
O. Y. Trofimova, I. V. Ershova, A. V. Maleeva, et al., Russ. J. Coord. Chem. 47, 610 (2021). https://doi.org/10.1134/S1070328421090086
H. X. Kang, Y. Q. Fu, L. Y. Xin, et al., Russ. J. Gen. Chem. 90, 2365 (2020). https://doi.org/10.1134/S107036322012021X
V. S. Gusarov, A. M. Cheplakova, D. G. Samsonenko, et al., Russ. J. Inorg. Chem. 66, 1374 (2021). https://doi.org/10.1134/S0036023621090035
Y. F. Wang, S. Q. Zhang, Y. X. Feng, et al., Russ. J. Gen. Chem. 91, 1566 (2021). https://doi.org/10.1134/S1070363221080193
V. N. Mikhaylov and I. A. Balova, Russ. J. Gen. Chem. 91, 2194 (2021). https://doi.org/10.1134/S1070363221110098
S. I. Pechenyuk, D. P. Domonov, and A. N. Gosteva, Russ. J. Gen. Chem. 91, 1834 (2021). https://doi.org/10.1134/S1070363221090310
J. Plešek, Chem. Rev. 92, 269 (1992). https://doi.org/10.1021/cr00010a005
F. Teixidor, C. Viñas, A. Demonceau, and R. Núñez, Pure Appl. Chem. 75, 1305 (2003). https://doi.org/10.1351/pac200375091305
I. B. Sivaev and V. I. Bregadze, in Organometallic Chemistry Research Perspectives, Ed. by R.P. Irwin (Nova Science Pub Inc., 2007).
I. B. Sivaev and V. I. Bregadze, Eur. J. Inorg. Chem. 11, 1433 (2009). https://doi.org/10.1002/ejic.200900003
Z. J. Leśnikowski, J. Med. Chem. 59, 7738 (2016). https://doi.org/10.1021/acs.jmedchem.5b01932
Funding
This work was carried out within the framework of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Science in the field of fundamental scientific research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Avdeeva
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Avdeeva, V.V., Kubasov, A.S., Korolenko, S.E. et al. Synthesis and Structures of Copper and Thallium(I) Coordination Compounds [Cu2[B10H10]]n and Tl2[B10H10] with the closo-Decaborate Anion. Russ. J. Inorg. Chem. 67, 628–635 (2022). https://doi.org/10.1134/S0036023622050023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622050023