Abstract
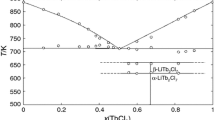
Multicomponent systems of alkali metal halides and chromates are used as molten electrolytes for chemical current sources, heat storage materials, etc. In this work, the ternary system RbF–RbBr–Rb2CrO4 was studied. The ternary systems МF–MBr–M2CrO4 (М = Li, Na, K, Rb, Cs) were analyzed by comparing the types of the liquidi of the systems in the series formed by successive replacement of the alkali metal with increasing its number in the periodic system. The RbF–RbBr–Rb2CrO4 system was investigated by differential thermal analysis. Phase equilibria in the system were explored; crystallizing phases were determined; and the characteristics of the ternary eutectic and the ternary peritectic were found to be (equiv. %) 39.5 RbF, 52.0 RbBr, 8.5 Rb2CrO4, the melting point 522°C and 19.7 RbF, 55.0 RbBr, 25.3 Rb2CrO4, the melting point 554°C, respectively.





Similar content being viewed by others
REFERENCES
E. I. Frolov, A. A. Finogenov, I. K. Garkushin, et al., Russ. J. Inorg. Chem. 65, 405 (2020). https://doi.org/10.31857/S0044457X20030034
G. E. Egortsev G.E. and I. K. Garkushin, Russ. J. Inorg. Chem. 53, 1495 (2008).
I. K. Garkushin and I. M. Kondratyuk, Analysis, Prediction and Experimental Study of Series of Systems of Halides of Alkali and Alkaline Earth Elements (UrO RAN, Ekaterinburg, 2006) [in Russian].
P. P. Fedorov, Russ. J. Inorg. Chem. 66, 550 (2021). https://doi.org/10.1134/S0036023621040100
P. A. Akhmedova, A. M. Gasanaliev, B. Y. Gamataeva. et al., Russ. J. Inorg. Chem. 62, 1390 (2017).
P. A. Akhmedova, A. M. Gasanaliev, B. Y. Gamataeva, et al., Russ. J. Inorg. Chem. 63, 837 (2018). https://doi.org/10.1134/S0036023618060025
A. R. Aliev, I. R. Akhmedov, M. G. Kakagasanov, et al., Russ. J. Phys. Chem. 92, 470 (2018).
A. S. Trunin, A. V. Budkin, and E. Yu. Moshchenskaya, Actual Problems of Modern Science, ch. 9 (Samara, 2003) [in Russian].
D. Mantha, T. Wang, and R. G. Reddy, J. Phase Equilibria Diffusion 33, 110 (2012). https://doi.org/10.1007/s11669-012-0005-4
L.-X. Jian, X.-Y. Wu, and Y.-Q. Tan, J. Hunan University Natural Sci. 41, 75 (2014).
I. K. Garkushin, T. V. Gubanova, E. I. Frolov, and E. Yu. Moshchenskaya, Elektrokhim. Energ. 10, 147 (2010).
P. Masset, J.-Y. Poinso, S. Schoeffert, et al., J. Electrochem. Soc. 152, A405 (2005). https://doi.org/10.1149/1.1850861
D. Sveinbjornsson, A. S. Christiansen, R. Viskinde, et al., J. Electrochem. Soc. 161, A1432 (2014). https://doi.org/10.1149/2.1061409jes
E. O. Ignat’eva and M. V. Chugunova, Ashirov. Chten. 1, 109 (2017).
E. O. Ignat’eva, E. M. Dvoryanova, and I. K. Garkushin, Kondens. Sredy Mezhfaz. Gran. 13, 445 (2011).
E. M. Dvoryanova, E. O. Ignat’eva, I. K. Garkushin, Butlerov Commun. 24, 71 (2011).
V. I. Posypaiko and E. A. Alekseeva, Melting Diagrams of Salt Systems, vol. III (Metallurgiya, Moscow, 1979) [in Russian].
N. K. Voskresenskaya, N. N. Evseeva, S. I. Berul’, and I. P. Vereshchatina, Fusibility Guide for Anhydrous Inorganic Salt Systems, ch. 1 (Izd-vo AN SSSR, Moscow, 1961) [in Russian].
M. Wagner, Thermal Analysis in Practice: Fundamental Aspects (Hanser Publications, 2018).
W. Wendlandt, Thermal Methods of Analysis (Interscience, 1964).
Yu. V. Moshchenskii, Prib. Tekh. Eksperim. 46, 143 (2003).
S. V. Fedotov, Yu. V. Moshchenskii, Interface Software DSCTool (Samar. Gos. Tekhn. Univ., Samara, 2004) [in Russian].
Thermal Constants of Substances. Handbook, Ed. by V. P. Glushko, vol. X, parts 1 and 2 (VINITI, Moscow) [in Russian].
A. S. Kosmynin and A. S. Trunin, Projection-Thermographic Method for Studying Heterogeneous Equilibria in Condensed Multicomponent Systems (Samar. Gos. Tekhn. Univ., Samara, 2006) [in Russian].
Funding
This work was supported by the Ministry of Science and Higher Education within a project section of State assignment no. 0778-2020-0005.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Kharchenko, A.V., Egorova, E.M. & Garkushin, I.K. Analysis of the Series of the Ternary Systems MF–MBr–M2CrO4 (M = Li, Na, K, Rb, Cs) and Experimental Investigation of the Ternary System RbF–RbBr–Rb2CrO4. Russ. J. Inorg. Chem. 67, 216–220 (2022). https://doi.org/10.1134/S0036023622020061
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023622020061