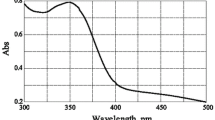
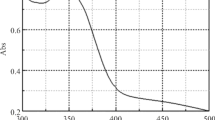
Abstract
The present paper deals with potentiometric study for formation of binary (1 : 1) and ternary (1 : 1 : 1) complexes of transition metals Ni(II), Cu(II), Co(II), and Zn(II) with Ceftriaxone Sodium and Esomeprazole in aqua-organic medium. The proton–ligand stability constant and metal–ligand stability constant has been calculated using Calvin–Bjerrum technique. The ionic strength has been maintained by 1.0 M NaClO4 and ternary complexes have been calculated by Thomson–Loraas method to determine log KMXY for all the ternary chelates.



Similar content being viewed by others
REFERENCES
A. Durrani, et al., Int. J. Chem. Stud. 1, 10 (2014).
E. V. Zhuravlev, V. G. Alekseev, M. A. Feofanova, and S. S. Ryasenskii, Russ. J. Inorg. Chem. 61, 877 (2016). https://doi.org/10.1134/S0036023616070214
A. A. Metlin, G. G. Gorboletova, S. A. Bychkova, and N. M. Berezina, Russ. J. Inorg. Chem. 62, 1116 (2017). https://doi.org/10.1134/S0036023617080149
A. Durrani and M. Jadhav, J. Med. Chem. Drug Discov., 2347 (2015)
S. Kitagawa, R.Kitaura, and S. I. Noro, Angew. Chem. Int. Ed. 43, 2334 (2004)
J. Akter, M. A. Hanif, M. S. Islam, et al., Chem. Sin. 8, 166 (2017).
A. R. Shaikh, S. Hussain, and M. Farooqui, J. Chem. Biol. Phys. Sci. 777, 267 (2016).
A. Durrani, et al., Uniq. Res. J. Chem. 2, 8 (2014).
R. P. Phase, A. G. Shankarwar, S. G. Shankarwar, and T. K. Chondhekar, Adv. Appl. Sci. Res. 4, 46 (2013).
D. B. Kamkhede, et al., Int. J. Res. Pharm. Chem. 6, 147 (2016).
S. V. Thakur, M. Farooqui, S. G. Shankarwar, and S. D. Naikwade, Int. J. Chem. Sci. 11, 464 (2013).
FDA Rocephin (cef. Sod.) for Injection available from: www.fda.gov/medwatch/safety/2007/safety07.htm, 2007.
M. Jambulingam, S. A. Thangathurai, D. Kamalakannan, et al., Pharm. Tutor. 3, 48 (2015).
T. M. Khomenko, K. P. Volcho, N. I. Komarova, and N. F. Salakhutdinov, Russ. J. Org. Chem. 44, 124 (2008). https://doi.org/10.1134/S1070428008010168
T. G. Rukari and G. V. Ahire, Asian J. Pharm. Clin. Res. 6, 121 (2013).
L. Olbe, E. Carlsson and P. Lindberg, Nat. Rev. Drug Discov. 2, 132 (2003). https://doi.org/10.1038/nrd1010
B. B. Tewari, et al., Russ. J. Inorg. Chem. 54, 151(2009). https://doi.org/10.1134/S0036023609010264
Z. Omar, S. Jadhav, M. Farooqui, and M. Rai, Int. J. Chemtech. Res. 11, 211 (2008). https://doi.org/10.20902/IJCTR.2018.111121
A. B. Naik and M. L. Narwade, Russ. J. Coord. Chem. 35, 932 (2009). https://doi.org/10.1134/S1070328409120136
L. C. Thompson and Loraas, Inorg. Chem. 2, 89 (1963). https://doi.org/10.1021/ic50005a025
J. K. Pathan, R. K. Pardeshi, M. Farooquiand, and D. M. Janrao, J. Med. Chem. Drug Discov. 2, 978 (2016).
S. C. Masikane, S. Mlowe, Russ. J. Inorg. Chem. 64, 1063 (2019). https://doi.org/10.1134/S0036023619080072
A. G. Beirakhov and A. V. Rotov, Russ. J. Inorg. Chem. 63, 1689 (2018). https://doi.org/10.1134/S0036023618130028
M. O. Karasev, I. N. Karaseva, and D. V. Pushkin, Russ. J. Inorg. Chem. 64, 870 (2019). https://doi.org/10.1134/S003602361907009X
M. A. Uvarova, A. A. Grineva, R. R. Datchuk, and S. E. Nefedov, Russ. J. Inorg. Chem. 63, 618 (2018). https://doi.org/10.1134/S0036023618050108
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sonone, A.V., Shaikh, M., Farooqui, M. et al. Potentiometric Studies of Binary and Ternary Complexes of Transition Metal Ions with Ceftriaxone Sodium and Esomeprazole. Russ. J. Inorg. Chem. 65, 390–394 (2020). https://doi.org/10.1134/S003602362003016X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S003602362003016X