Abstract
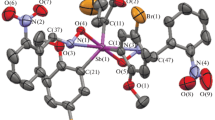
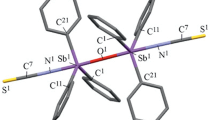
The tris(5-bromo-2-methoxyphenyl)antimony complexes with 2,3,4,5-tetrafuorobenzoic, bromodifluoroacetic, and heptafluorobutyric acids have been synthesized by oxidative addition reactions and structurally characterized. According to X-ray diffraction data, the products of these reactions are triarylantimony dicarboxylates with the general formula (2-MeO-5-Br-C6H3)3Sb(OC(O)R)2, where R = C6HF4-2,3,4,5 (1), CF2Br (2), CF2CF2CF3 (3). The antimony atoms in molecules of complexes 1–3 have trigonal bipyramidal coordination with the oxygen atoms of carboxylate ligands in axial positions. The average lengths are 2.119 (1), 2.106 (2), and 2.109 Å (3) for equatorial Sb–C bonds and 2.112 (1), 2.104 (2), and 2.118 Å (3) for Sb–O bonds. The ОSbО angles are 177.5(2)° (1), 172.6(4)° (2), and 176.8(3)° (3), and the OSbC angles are 89.3(3)°–93.5(3)° (1), 84.4(5)°–97.0(5)° (2), and 84.2(3)°–98.9(3)° (3). The photocatalytic activity of the synthesized complexes has been studied using the photodestruction of organic dyes, namely, Methylene Blue and Methyl Violet as an example.









Similar content being viewed by others
REFERENCES
T. Iftikhar, M. K. Rauf, S. Sarwar, et al., J. Organomet. Chem. 851, 89 (2017). https://doi.org/10.1016/j.jorganchem.2017.09.002
L. Yu, Y. Q. Ma, G. C. Wang, and J. S. Li, Heteroat. Chem. 15, 32 (2004). https://doi.org/10.1002/hc.10208
L. Saleem, A. A. Altaf, A. Badshah, et al., Inorg. Chim. Acta 474, 148 (2018). https://doi.org/10.1016/j.ica.2018.01.036
R. Mushtaq, M. K. Rauf, M. Bolte, et al., Appl. Organomet. Chem. 30, 1 (2016). https://doi.org/10.1002/aoc.3456
R. Mushtaq, M. K. Rauf, M. Bond, et al., Appl. Organomet. Chem. 31, 1 (2015). https://doi.org/10.1002/aoc.3606
A. Islam, J. G. Da Silva, and F. M. Berbet, Molecules 19, 6009 (2014). https://doi.org/10.3390/molecules19056009
M. I. Ali, M. K. Rauf, A. Badshah, et al., J. Chem. Soc., Dalton Trans. 42, 16733 (2013). https://doi.org/10.1039/c3dt51382c
Y. Q. Ma, L. Yu, and J. S. Li, Heteroat. Chem. 13, 299 (2002). https://doi.org/10.1002/hc.10033
P. Sharma, D. Perez, A. Cabrera, et al., Acta Pharm. Sin. 29, 881 (2008). https://doi.org/10.1111/j.1745-7254.2008.00818.x
S. Abdolmaleki, S. Yarmohammadi, N. Adib, et al., Polyhedron 159, 239 (2019). https://doi.org/10.1016/J.POLY.2018.11.063
H. X. Qi, H. Jo, H. E. Lee, et al., J. Solid State Chem. 274, 69 (2019). https://doi.org/10.1016/J.JSSC.2019.03.018
X. Y. Zhang, L. S. Cui, X. Zhang, et al., J. Mol. Struct. 1134, 742 (2017). https://doi.org/10.1016/j.molstruc.2017.01.039
S. Agnihotri, P. Raj, and K. Singhal, Synt. React. Inorg. Met.-Org. Chem. 32, 449 (2002). https://doi.org/10.1081/SIM-120003788
H. Geng, M. Hong, Y. Yang, et al., J. Coord. Chem. 68 2938 (2015). https://doi.org/10.1080/00958972.2015.1060322
L. Wen, H. Yin, W. Li, et al., Inorg. Chim. Acta 363, 676 (2010). https://doi.org/10.1016/j.ica.2009.11.022
L. Yu, Y. Q. Ma, R. C. Liu, et al., Polyhedron 23, 823 (2004). https://doi.org/10.1016/j.poly.2003.12.002
R. N. Duffin, V. L. Blair, L. Kedzierski, et al., J. Chem. Soc., Dalton Trans. 47, 971 (2018). https://doi.org/10.1039/C7DT04171C
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. J. Gen. Chem. 82, 95 (2012). https://doi.org/10.1134/S1070363212010161
V. V. Sharutin, O. K. Sharutina, R. V. Reshetnikova, et al., Russ. J. Inorg. Chem. 62, 1450 (2017). https://doi.org/10.1134/S003602361711016X
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. J. Inorg. Chem. 56, 1561 (2011). https://doi.org/10.1134/S0036023611100196
SMART: Bruker Molecular Analysis Research Tool, Versions 5.625 (Bruker, Madison, WI, 2000).
SAINTPlus: Data Reduction and Correction Program, Versions 6.02a (Bruker, Madison, WI, 2000).
SHELXTL/PC: An Integrated System for Solving, Refining and Displaying Crystal Structures from Diffraction Data, Versions 5.10 (Bruker, Madison, WI, 1998).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, et al., J. Appl. Crystallogr. 42, 339 (2009).
G. O. Doak, G. G. Long, and L. D. Freedman, J. Organomet. Chem. 4, 82 (1965). https://doi.org/10.1016/S0022-328X(0000)823700-0
A. Gupta, R. K. Sharma, R. Bohra, et al., Polyhedron 21 (23), 2387 (2002). https://doi.org/10.1016/S0277-5387(02)01155-5
V. V. Sharutin, V. S. Senchurin, O. K. Sharutina, et al., Russ. J. Coord. Chem. 37, 781 (2011). https://doi.org/10.1134/S1070328411090089
V. V. Sharutin, O. K. Sharutina, V. S. Senchurin, and O. V. Chagarova, Russ. J. Gen. Chem. 82, 1665 (2012). https://doi.org/10.1134/S1070363212100064
S. Tothadi, S. Joseph, and G. R. Desiraju, Cryst. Growth Des. 13, 3242 (2013). https://doi.org/10. 1021/cg400735f
V. M. Muzalevskiy, A. M. Magerramov, and N. G. Shihaliev, et al., Russ. Chem. Bull. 65, 1541 (2016). https://doi.org/10.1007/s11172-016-1480-2
J. A. Smith, M. A. Singh-Wilmot, K. P. Carter, et al., Cryst. Growth Des. 19, 305 (2019). https://doi.org/10.1021/acs.cgd.8b01426
K. Shimizu and J. F. da Silva, Molecules 23, 2959 (2018). https://doi.org/10.3390/molecules23112959
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within state task no. 4.6151.2017/8.9.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Glushachenkova
Rights and permissions
About this article
Cite this article
Artem’eva, E.V., Sharutina, O.K., Sharutin, V.V. et al. Complexes Ar3Sb[OC(O)C6HF4-2,3,4,5]2, Ar3Sb[OC(O)CF2Br]2, and Ar3Sb[OC(O)CF2CF2CF3]2 (Ar = C6H3OMe-2-Br-5): Synthesis, Structure, and Photochemical Properties. Russ. J. Inorg. Chem. 65, 22–29 (2020). https://doi.org/10.1134/S0036023620010039
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620010039