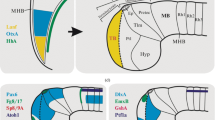
Abstract
The basic knowledge about the development and formation of the body plan of vertebrates (Vertebrata) was obtained while working with traditional and generally accepted model objects, such as the embryos of mice (Muridae), chickens (Gallus), bony fish (Teleostei), clawed frogs (Xenopus). At the same time, for understanding the evolutionary history of vertebrates, studies of so-called “non-model” objects are of particular value. These animals represent important phylogenetic branches on the evolutionary tree of vertebrates, but do not possess a set of qualities that make them convenient for laboratory work. First of all, such objects include the most ancient living representatives of the taxa, basally diverged from the common evolutionary trunk. These are cyclostomes (Cyclostomata) in the case of vertebrates in general, cartilaginous fish (Chondrichthyes) in the case of gnathostomes (Gnathostomata) and bone ganoids (Holostei) as representatives of new-finned fish (Neopterygii). The research value of these ancient groups lies in the fact that morphological features that are later characteristic of the entire taxon appear in their evolution for the first time, for example, the telencephalon and neural crest cells in cyclostomes, paired fins and the jaws in cartilaginous fish. Representatives of these groups provide an opportunity to study the evolutionary premieres of individual structures and features and mechanisms that ensured their emergence. This article presents an overview of studies of whole-genome duplications at the early stages of vertebrate evolution and the emergence of a unique forebrain region, the telencephalon, which we studied on lampreys, as the most ancient representatives of vertebrates available for laboratory research today.





Similar content being viewed by others
REFERENCES
Bayramov, A.V., Martynova, N.Yu., Eroshkin, F.M., et al., The homeodomain-containing transcription factor X-nkx-5.1 inhibits expression of the homeobox gene Xanf-1 during the Xenopus laevis forebrain development, Mech. Devel., 2004, vol. 121, no. 12, pp. 1425–1441. https://doi.org/10.1016/j.mod.2004.08.002
Bayramov, A.V., Eroshkin, F.M., Martynova, N.Y., et al., Novel functions of Noggin proteins: inhibition of Activin/Nodal and Wnt signaling, Development, 2011, vol. 138, no. 6, pp. 5345–5356. https://doi.org/10.1242/dev.068908
Bayramov, A.V., Ermakova, G.V., Eroshkin, F.M., et al., The presence of the Anf/Hesx1 homeobox in lampreys indicates that it may play important role in telencephalon emergence, Sci. Rep., 2016, vol. 6, no. 1, art. ID 39849. https://doi.org/10.1038/srep39849
Bayramov, A.V., Ermakova, G.V., Eroshkin, F.M., et al., Finding of the homeobox gene Anf in lamprey Lethenteron camtschaticum support the hypothesis that emergence of telencephalon in vertebrates evolution was due to the advent of Anf genes, Russ. J. Devel. Biol., 2017, vol. 48, no. 4, pp. 241–251. https://doi.org/10.7868/S0475145017040024
Bayramov, A.V., Ermakova, G.V., Kucheryavyy, A.V., and Zaraisky, A.G., Lampreys—“living fossils” in researches of early development and regeneration of the vertebrates, Russ. J. Devel., 2018, vol. 49, no. 6, pp. 327–338. https://doi.org/10.1134/S1062360418080015
Bayramov, A.V., Ermakova, G.V., and Zaraisky, A.G., Genetic mechanisms of the early development of the telencephalon, a unique segment of the vertebrate central nervous system, as reflecting its emergence and evolution, Russ. J. Devel., 2020, vol. 51, no. 3, pp. 162–175. https://doi.org/10.1134/S1062360420030054
Bayramov, A.V., Ermakova, G.V., Kuchryavyy, A.V., and Zaraisky A.G., Genome duplications as the basis of vertebrates’ evolutionary success, Russ. J. Devel., 2021, vol. 52, no. 3, pp. 141–163. https://doi.org/10.1134/S1062360421030024
Braasch, I., Gehrke, A.R., Smith, J.J., et al., The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons, Nat. Genet., 2015, vol. 47, no. 4, pp. 427–437. https://doi.org/10.1038/ng.3526
Cardoso, J.C.R., Bergqvist, C.A., Félix, R.C., et al., Corticotropin-releasing hormone family evolution: five ancestral genes remain in some lineages, J. Mol. Endocrinol., 2016, vol. 57, no. 1, pp. 73–86. https://doi.org/10.1530/JME-16-0051
Cardoso, J.C.R., Bergqvist, C.A., and Larhammar, D., Corticotropin-releasing hormone (CRH) gene family duplications in lampreys correlate with two early vertebrate genome doublings, Front. Neurosci., 2020, vol. 14, art. ID 672. https://doi.org/10.3389/fnins.2020.00672
Carroll, S.B., Homeotic genes and the evolution of arthropods and chordates, Nature, 1995, vol. 376, no. 6540, pp. 479–485. https://doi.org/10.1038/376479a0
Cohen, A.H., Mackler, S.A., and Selzer, M.E., Functional regeneration following spinal transection demonstrated in the isolated spinal cord of the larval Sea lamprey, Proc. Natl. Acad. Sci., 1986, vol. 83, no. 8, pp. 2763–2766. https://doi.org/10.1073/pnas.83.8.2763
Dahn, R.D., Davis, M.C., Pappano, W.N., and Shubin, N.H., Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning, Nature, 2007, vol. 445, no. 7125, pp. 311–314. https://doi.org/10.1038/nature05436
Dale, L. and Slack, J.M.W., Regional specification within the mesoderm of early embryos of Xenopus laevis, Development, 1987, vol. 100, no. 2, pp. 279–295. https://doi.org/10.1242/dev.100.2.279
Danesin, C. and Houart, C., A Fox stops the Wnt: implications for forebrain development and diseases, Curr. Opin. Genet. Dev., 2012, vol. 22, no. 4, pp. 323–330. https://doi.org/10.1016/j.gde.2012.05.001
Danesin, C., Peres, J.N., Johansson, M., et al., Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1, Dev Cell., 2009, vol. 16, no. 4, pp. 576–587. https://doi.org/10.1016/j.devcel.2009.03.007
Donoghue, P.C. and Keating, J.N., Early vertebrate evolution, Palaeontology, 2014, vol. 57, no. 5, pp. 879–893. https://doi.org/10.1111/pala.12125
Donoghue, P.C.J. and Purnell, M.A., Genome duplication, extinction and vertebrate evolution, Trends Ecol. Evol., 2005, vol. 20, no. 6, pp. 312–319. https://doi.org/10.1016/j.tree.2005.04.008
Ermakova, G.V., Alexandrova, E.M., Kazanskaya, O.V., et al., The homeobox gene, Xanf-1, can control both neural differentiation and patterning in the presumptive anterior neurectoderm of the Xenopus laevis embryo, Development, 1999, vol. 126, no. 20, pp. 4513–4523. https://doi.org/10.1242/dev.126.20.4513
Ermakova, G.V., Solovieva, E.A., Martynova, N.Y., et al., The homeodomain factor Xanf represses expression of genes in the presumptive rostral forebrain that specify more caudal brain regions, Devel. Biol., 2007, vol. 307, no. 2, pp. 483–497. https://doi.org/10.1016/j.ydbio.2007.03.524
Ermakova, G.V., Kucheryavyy, A.V., Zaraisky, A.G., and Bayramov, A.V., The expression of FoxG1 in the early development of the European river lamprey Lampetra fluviatilis demonstrates significant heterochrony with that in other vertebrates, Gene Expr. Patterns, 2019, vol. 28, no. 34, art. ID 119073. https://doi.org/10.1016/j.gep.2019.119073
Ermakova, G.V., Kucheryavyy, A.V., Zaraisky, A.G., and Bayramov, A.V., Discovery of four Noggin genes in lampreys suggests two rounds of ancient genome duplication, Commun. Biol., 2020a, vol. 3, no. 1, art. ID 532. https://doi.org/10.1038/s42003-020-01234-3
Ermakova, G.V., Kucheryavyy, A.V., Zaraisky, A.G., and Bayramov, A.V., Heterochrony of the expression of Lanf and Foxg1 in lamprey confirms the appearance of the telencephalon as an evolutionarily young superstructure in the central nervous system of vertebrates, Russ. J. Devel. Biol., 2020b, vol. 51, no. 4, pp. 246–254. https://doi.org/10.1134/S1062360420040049
Ermakova, G.V., Kucheryavyy, A.V., Eroshkin, F.M., et al., Study of the early telencephalon genes of cyclostomes as a way to restoring the evolutionary history of this unique part of the central nervous system of vertebrates, Paleontol. J., 2021a, vol. 55, no. 7, pp. 38–51. https://doi.org/10.1134/S0031030121070030
Ermakova, G.V., Kucheryavyy, A.V., Zaraisky, A.G., and Bayramov, A.V., Comparative analysis of expression patterns of genes of the Noggin family at early stages of development of the head structures of the European river lamprey Lampetra fluviatilis, Russ. J. Devel. Biol., 2021b, vol. 52, no. 1, pp. 33–41. https://doi.org/10.1134/S1062360421010033
Eroshkin, F., Kazanskaya, O., Martynova, N., et al., Characterization of cis-regulatory elements of the homeobox gene Xanf-1, Gene, 2002, vol. 285, no. 1–2, pp. 279–286. https://doi.org/10.1016/s0378-1119(02)00393-1
Eroshkin, F.M., Ermakova, G.V., Bayramov, A.V., et al., Multiple noggins in vertebrate genome: cloning and expression of noggin2 and noggin4 in Xenopus laevis, Gene Expr. Patterns, 2006. vol. 6, no. 2, p. 180–186. https://doi.org/10.1016/j.modgep.2005.06.007
Evans, T.M., Janvier, P., and Docker, M.F., The evolution of lamprey (Petromyzontida) life history and the origin of metamorphosis, Rev. Fish Biol. Fish., 2018, vol. 28, no. 4, pp. 825–838. https://doi.org/10.1007/s11160-018-9536-z
Feinberg, T.E. and Mallatt, J., The evolutionary and genetic origins of consciousness in the Cambrian Period over 500 million years ago, Front. Psychol., 2013, vol. 4, art. ID 667. https://doi.org/10.3389/fpsyg.2013.00667
Fletcher, R.B., Watson, A.L., and Harland, R.M., Expression of Xenopus tropicalis noggin1 and noggin2 in early development: two noggin genes in a tetrapod, Gene Expr. Patterns, 2004, vol. 5, no. 2, pp. 225–230. https://doi.org/10.1016/j.modgep.2004.08.001
Fritzsch, B., Similarities and differences in lancelet and craniate nervous systems, Isr. J. Zool., 1996, vol. 42, Suppl. 1, pp. S147–S160. https://www.tandfonline.com/doi/abs/ 10.1080/00212210.1996.10688878
Gee, H., Across the Bridge: Understanding the Origin of the Vertebrates, Chicago: Univ. Chicago Press, 2018.
Gess, R.W., Coates, M.I., and Rubidge, B.S., A lamprey from the Devonian period of South Africa, Nature, 2006, vol. 443, no. 7114, pp. 981–984. https://doi.org/10.1038/nature05150
Gilbert, S.F., Developmental Biology, Sunderland: Sinauer Associates, 2006.
Graham, A., Papalopulu, N., and Krumlauf, R., The murine and Drosophila homeobox gene complexes have common features of organization and expression, Cell, 1989, vol. 57, no. 3, pp. 367–378. https://doi.org/10.1016/0092-8674(89)90912-4
Green, S.A. and Bronner, M.E., The lamprey: A jawless vertebrate model system for examining origin of the neural crest and other vertebrate traits, Differentiation, 2014, vol. 87, no. 1–2, pp. 44–51. https://doi.org/10.1016/j.diff.2014.02.001
Heimberg, A.M., Cowper-Sal-Lari, R., Sémon M., et al., microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate, Proc. Natl. Acad. Sci. USA, 2010, vol. 107, no. 45, pp. 19379–19383. https://doi.org/10.1073/pnas.1010350107
Herman, P.E., Papatheodorou, A., Bryant, S.A., et al. Highly conserved molecular pathways, including Wnt signaling, promote functional recovery from spinal cord injury in lampreys, Sci. Rep., 2018, vol. 8, no. 1. art. ID 742. https://doi.org/10.1038/s41598-017-18757-1
Holland, L.Z. and Ocampo Daza, D., A new look at an old question: when did the second whole genome duplication occur in vertebrate evolution?, Genome Biol., 2018, vol. 19, no. 1, art. ID 209. https://doi.org/10.1186/s13059-018-1592-0
Holland, L.Z. and Short, S., Gene duplication, co-option and recruitment during the origin of the vertebrate brain from the invertebrate chordate brain, Brain Behav. Evol., 2008, vol. 72, pp. 91–105. https://doi.org/10.1159/000151470
Hume, J.B., Adams, C.E., Mable, B., and Bean, C, Post-zygotic hybrid viability in sympatric species pairs: a case study from European lampreys, Biol. J. Linn. Soc., 2013, vol. 108, no. 2, pp. 378–383. https://doi.org/10.1111/j.1095-8312.2012.02007.x
Irimia, M., Pineiro, C., Maeso, I., et al., Conserved developmental expression of Fezf in chordates and Drosophila and the origin of the Zona Limitans Intrathalamica (ZLI) brain organizer, Evodevo, 2010, vol. 1, no. 1, art. ID 7. https://doi.org/10.1186/2041-9139-1-7
Janvier, P., The dawn of the vertebrates: characters versus common ascent in the rise of current vertebrate phylogenies, Palaeontology, 1996, vol. 39, Part 2, pp. 259–287.
Janvier, P., Vertebrate characters and the Cambrian vertebrates, C.R. Palevol., 2003, vol. 2, no. 6–7, pp. 523–531. https://doi.org/10.1016/j.crpv.2003.09.002
Janvier, P., Modern look for ancient lamprey, Nature, 2006, vol. 433, no. 7114, pp. 921–924. https://doi.org/10.1038/443921a
Janvier, P., Facts and fancies about early fossil chordates and vertebrates, Nature, 2015, vol. 520, no. 7548, pp. 483–489. https://doi.org/10.1038/nature14437
Klimova, L. and Kozmik, Z., Stage-dependent requirement of neuroretinal Pax6 for lens and retina development, Development, 2014, vol. 141, no. 6, pp. 1292–1302. https://doi.org/10.1242/dev.098822
Kortüm, F., Das, S., Flindt, M., et al., The core FOXG1 syndrome phenotype consists of postnatal microcephaly, severe mental retardation, absent language, dyskinesia, and corpus callosum hypogenesis, J. Med. Genet., 2011, vol. 8, no. 6, pp. 396–406. https://doi.org/10.1136/jmg.2010.087528
Kumamoto, T. and Hanashima, C., Evolutionary conservation and conversion of Foxg1 function in brain development, Devel. Growth Differ., 2017, vol. 59, no. 4, pp. 258–269. https://doi.org/10.1111/dgd.12367
Kuraku, S. and Kuratani, S., Time scale for cyclostome evolution inferred with a phylogenetic diagnosis of hagfish and lamprey cDNA sequences, Zool. Sci., 2006, vol. 23, no. 12, pp. 1053–1064. https://doi.org/10.2108/zsj.23.1053
Kuratani, S. and Ota, K.G., Hagfish (Cyclostomata, Vertebrata): searching for the ancestral developmental plan of vertebrates, BioEssays, 2008, vol. 30, no. 2, pp. 167–172. https://doi.org/10.1002/bies.20701
Kuratani, S., Nobusada, Y., Horigome, N., et al., Embryology of the lamprey and evolution of the vertebrate jaws: insights from molecular and developmental perspectives, Philos. Trans. R. Soc. Lond., B, Biol. Sci., 2001, vol. 356, no. 1414, pp. 1615–1632. https://doi.org/10.1098/rstb.2001.0976
Lamb, T.M., Knecht, A.K., Smith, W.C., et al., Neural induction by secreted polypeptide noggin, Science, 1993, vol. 262, no. 5134, pp. 713–718. https://doi.org/10.1126/science.8235591
Martynova, N.Yu., Eroshkin, F.M., Ermakova, G.V., et al., Patterning the forebrain: FoxA4a/Pintallavis and Xvent-2 determine the posterior limit of the Xanf-1 expression in the neural plate, Development, 2004, vol. 131, no. 10, pp. 2329–2338. https://doi.org/10.1242/dev.01133
Martynoga, B., Morrison, H., Price, D.J., et al., Foxg1 is required for specification of ventral telencephalon and region-specific regulation of dorsal telencephalic precursor proliferation and apoptosis, Devel. Biol., 2005, vol. 283, no. 1, pp. 113–127. https://doi.org/10.1016/j.ydbio.2005.04.005
McCauley, D.W., Docker, M.F., Whyard, S., et al., Lampreys as diverse model organisms in the genomics era, Bioscience, 2015, vol. 65, no. 11, pp. 1046–1056. https://doi.org/10.1093/biosci/biv139
Mehta, T.K., Ravi, V., Yamasaki, S., et al., Evidence for at least six Hox clusters in the Japanese lamprey (Lethenteron japonicum), Proc. Natl. Acad. Sci. USA, 2013, vol. 110, no. 40, pp. 16044–16049. https://doi.org/10.1073/pnas.1315760110
Meléndez-Ferro, M., Villar-Cheda, B., Abalo, X.M., et al., Early development of the retina and pineal complex in the Sea lamprey: comparative immunocytochemical study, J. Comp. Neurol., 2002, vol. 442, no. 3, pp. 250–265. https://doi.org/10.1002/cne.10090
Moreau, M. and Leclerc, C., The choice between epidermal and neural fate: a matter of calcium, Int. J. Devel. Biol., 2004, vol. 48, no. 2–3, pp. 75–84. https://doi.org/10.1387/ijdb.15272372
Murakami, Y., Uchida, K., Rijli, F.M., and Kuratani, S., Evolution of the brain developmental plan: Insights from agnathans, Dev. Biol., 2005, vol. 280, no. 2, p. 249–259. https://doi.org/10.1016/j.ydbio.2005.02.008
Nakatani, Y., Shingate, P., Ravi, V., et al. Reconstruction of proto-vertebrate, proto-cyclostome and proto-gnathostome genomes provides new insights into early vertebrate evolution, Nat. Commun., 2021, vol. 12, no. 1, art. ID 4489. https://doi.org/10.1038/s41467-021-24573-z
Noro, M., Sugahara, F., and Kuraku, S., Reevaluating Emx gene phylogeny: homopolymeric amino acid tracts as a potential factor obscuring orthology signals in cyclostome genes, BMC Evol. Biol., 2015, vol. 15, no. 1, art. ID 78. https://doi.org/10.1186/s12862-015-0351-z
Ohno, S., Evolution by Gene Duplication, Heidelberg: Springer Berlin, 1970. https://doi.org/10.1007/978-3-642-86659-3
Oisi, Y., Ota, K.G., and Kuraku, S., Craniofacial development of hagfishes and the evolution of vertebrates, Nature, 2013, vol. 493, no. 7431, pp. 175–180. https://doi.org/10.1038/nature11794
Onimaru, K. and Kuraku, S., Inference of the ancestral vertebrate phenotype through vestiges of the whole-genome duplications, Brief Funct. Genomics, 2018, vol. 17, no. 5, pp. 352–361. https://doi.org/10.1093/bfgp/ely008
Osório, J. and Rétaux, S., The lamprey in evolutionary studies, Devel. Genes. Evol., 2008, vol. 218, no. 5, pp. 221–235. https://doi.org/10.1007/s00427-008-0208-1
Osumi, N., Shinohara, H., Numayama-Tsuruta, K., et al., Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator, Stem. Cells, 2008, vol. 26, no. 7, p. 1663–1672. https://doi.org/10.1634/stemcells.2007-0884
Ota, K.G., Kuraku, S., and Kuratani, S., Hagfish embryology with reference to the evolution of the neural crest, Nature, 2007, vol. 446, no. 7136, pp. 672–675. https://doi.org/10.1038/nature05633
Parker, H.J., Bronner, M.E., and Krumlauf, R., An atlas of anterior hox gene expression in the embryonic sea lamprey head: Hox-code evolution in vertebrates, Devel. Biol., 2019, vol. 453, no. 1, pp. 19–33. https://doi.org/10.1016/j.ydbio.2019.05.001
Pasquier, J., Braasch, I., Batzel, P., et al., Evolution of gene expression after whole-genome duplication: new insights from the spotted gar genome, J. Exp. Zool., B, Mol. Devel. Evol., 2017, vol. 328, no. 7, pp. 709–721. https://doi.org/10.1002/jez.b.22770
Pavlov, D.S., Nazarov, D.Y., Zvezdin, A.O., et al. Downstream migration of early larvae of the European river lamprey Lampetra fluviatilis, Dokl. Biol. Sci., 2014, vol. 459, no. 1, pp. 344–347. https://doi.org/10.1134/S0012496614060039
Pereira, A., Levy, A., Vukić, J., et al., Putting European lampreys into perspective: A global-scale multilocus phylogeny with a proposal for a generic structure of the Petromyzontidae, J. Zool. Syst. Evol. Res., 2021. vol. 59, no. 8, pp. 1982–1993. https://doi.org/10.1111/jzs.12522
Piavis, G.W., Embryological stages in the sea lamprey and effects of temperature on development, U.S. Fish Wildl. Serv. Biol. Bull., 1961, vol. 61, no. 182, pp. 111–143.
Qiu, H., Hildebrand, F., Kuraku, S., et al., Unresolved orthology and peculiar coding sequence properties of lamprey genes: the KCNA gene family as test case, BMC Genomics, 2011, vol. 12, no. 1, art. ID 325. https://doi.org/10.1186/1471-2164-12-325
Ravi, V., Bhatia, S., Shingate, P., et al., Lampreys, the jawless vertebrates, contain three Pax6 genes with distinct expression in eye, brain and pancreas, Sci. Rep., 2019, vol. 9, no. 1, art. ID 19559. https://doi.org/10.1038/s41598-019-56085-8
Renaud, C.B., Lampreys of the world. An annotated and illustrated catalogue of lamprey species known to date, FAO Species Cat. Fish. Purp., no. 5. Rome: FAO, 2011.
Reyes, R.C., Embryogenesis and ammocoete morphological development of the Pacific lamprey (Entosphenus tridentatus Gairdner, 1836) from the American River, California, Tracy Tech. Bull., 2008, vol. 3.
Richardson, M.K. and Wright, G.M., Developmental transformations in a normal series of embryos of the sea lamprey Petromyzon marinus (Linnaeus), J. Morph., 2003, vol. 257, no. 3, pp. 348–363. https://doi.org/10.1002/jmor.10119
Richardson, M.K., Admiraal, J., and Wright, G.M., Developmental anatomy of lampreys, Biol. Rev. Camb. Philos. Soc., 2010, vol. 85, no. 1, pp. 1–33. https://doi.org/10.1111/j.1469-185X.2009.00092.x
Sacerdot, C., Louis, A., Bon, C., et al., Chromosome evolution at the origin of the ancestral vertebrate genome, Genome Biol., 2018, vol. 19, no. 1, p. 166. https://doi.org/10.1186/s13059-018-1559-1
Sharpe, C.R., Fritz, A., De Robertis, E.M., et al., A homeobox-containing marker of posterior neural differentiation shows the importance of predetermination in neural induction, Cell, 1987, vol. 50, no. 5, pp. 749–758. https://doi.org/10.1016/0092-8674(87)90333-3
Shimeld, S.M. and Donoghue, P.C.J., Evolutionary crossroads in developmental biology: cyclostomes (lamprey and hagfish), Development, 2012, vol. 139, no. 12, pp. 2091–2099. https://doi.org/10.1242/dev.074716
Simakov, O., Marlétaz, F., Yue, J.X., et al., Deeply conserved synteny resolves early events in vertebrate evolution, Nat. Ecol. Evol., 2020, vol. 4, no. 6, pp. 820–830. https://doi.org/10.1038/s41559-020-1156-z
Slack, J.M. and Tannahill, D., Noggin the dorsalizer, Nature, 1993, vol. 361, no. 6412, pp. 498–499. https://doi.org/10.1038/361498a0
Smith, A.J., Howell, J.H., and Piavis, G.W., Comparative embryology of five species of lamprey of the Upper Great Lakes, Copeia, 1968, vol. 1968, no. 3, pp. 461–469. https://doi.org/10.2307/1442013
Smith, J.J., Kuraku, S., Holt, C., et al., Sequencing of the sea lamprey (Petromyzon marinus) genome provides insights into vertebrate evolution, Nat. Genet., 2013, vol. 45, no. 4, pp. 415–421. https://doi.org/10.1038/ng.2568
Smith, J.J. and Keinath, M.C., The sea lamprey meiotic map improves resolution of ancient vertebrate genome duplications, Genome Res., 2015, vol. 25, no. 8, pp. 1081–1090. https://doi.org/10.1101/gr.184135.114
Smith, J.J., Timoshevskaya, N., Ye, C., et al., The sea lamprey germline genome provides insights into programmed genome rearrangement and vertebrate evolution, Nat. Genet., 2018, vol. 50, no. 2, pp. 270–277. https://doi.org/10.1038/s41588-017-0036-1
Smith, W.C. and Harland, R.M., Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos, Cell, 1992, vol. 70, no. 5, pp. 829–840. https://doi.org/10.1016/0092-8674(92)90316-5
Smith, W.C., Knecht, A.K., Wu, M., et al., Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm, Nature, 1993, vol. 361, no. 6412, pp. 547–549. https://doi.org/10.1038/361547a0
Square, T., Romášek, M., Jandzik, D., et al., CRISPR/Cas9-mediated mutagenesis in the Sea lamprey Petromyzon marinus: a powerful tool for understanding ancestral gene functions in vertebrates, Development, 2015, vol. 142, no. 23, pp. 4180–4187. https://doi.org/10.1242%2Fdev.125609
Suda, Y., Kurokawa, D., Takeuchi, M., et al., Evolution of Otx paralogue usages in early patterning of the vertebrate head, Devel. Biol., 2009, vol. 325, no. 1, pp. 282–295. https://doi.org/10.1016/j.ydbio.2008.09.018
Sugahara, F., Murakami, Y., and Kuratani, S., Gene Expression Analysis of Lamprey Embryo, New York: Springer, 2015.
Sugahara, F., Pascual-Anaya, J., Oisi, Y., et al., Evidence from cyclostomes for complex regionalization of the ancestral vertebrate brain, Nature, 2016, vol. 531, no. 7592, pp. 97–100. https://doi.org/10.1038/nature16518
Sugahara, F., Murakami, Y., Pascual-Anaya, J., et al., Reconstructing the ancestral vertebrate brain, Devel. Growth Differ., 2017, vol. 59, no. 4, pp. 163–174. https://doi.org/10.1111/dgd.12347
Suzuki, D.G., Murakami, Y., Escriva, H., et al., A comparative examination of neural circuit and brain patterning between the lamprey and amphioxus reveals the evolutionary origin of the vertebrate visual center, 2015, J. Comp. Neurol., vol. 523, no. 2, pp. 251–261. https://doi.org/10.1002/cne.23679
Tahara, Y., Normal stages of development in the lamprey, Lampetra reissneri (Dybowski), Zool. Sci., 1988, vol. 5, pp. 109–118.
Tank, E.M., Dekker, R.G., Beauchamp, K., et al., Patterns and consequences of vertebrate Emx gene duplications, Evol. Devel., 2009, vol. 11, no. 4, pp. 343–353. https://doi.org/10.1111/j.1525-142X.2009.00341.x
Tomsa, J.M. and Langeland, J.A., Otx expression during lamprey embryogenesis provides insights into the evolution of the vertebrate head and jaw, Devel. Biol., 1999, vol. 207, no. 1, pp. 26–37. https://doi.org/10.1006/dbio.1998.9163
Toresson, H., Martinez-Barbera, J.P., Bardsley, A., et al., Conservation of BF-1 expression in amphioxus and zebrafish suggests evolutionary ancestry of anterior cell types that contribute to the vertebrate telencephalon, Devel. Genes Evol., 1998, vol. 208, no. 8, pp. 431–439. https://doi.org/10.1007/s004270050200
Tsimbalov, I.A., Kucheryuavyi, A.V., and Pavlov, D.S., Results of Hybridization between Anadromous and Resident Forms of European River Lamprey Lampetra fluviatilis, J. Ichthyol., 2018, vol. 58, no. 1, pp. 122–125. https://doi.org/10.1134/S0032945218010137
Van de Peer, Y., Mizrachi, E., and Marchal, K., The evolutionary significance of polyploidy, Nat. Rev. Genet., 2017, vol. 18, no. 7, pp. 411–424. https://doi.org/10.1038/nrg.2017.26
Venkatesh, T.V., Holland, N.D., Holland, L.Z., et al., Sequence and developmental expression of amphioxus AmphiNk2-1: insights into the evolutionary origin of the vertebrate thyroid gland and forebrain, Devel. Genes Evol., 1999, vol. 209, no. 4, pp. 254–259. https://doi.org/10.1007/s004270050250
Xanthos, J.B., Kofron, M., Tao, Q., et al., The roles of three signaling pathways in the formation and function of the Spemann Organizer, Development, 2002, vol. 129, no. 17, pp. 4027–4043. https://doi.org/10.1242/dev.129.17.4027
Yang, X.U., Si-Wei, Z.H.U., Qing-Wei, L.I., Lamprey: a model for vertebrate evolutionary research, Dongwuxue Yanjiu, 2016, vol. 37, no. 5, p. 263–269. https://doi.org/10.13918/j.issn.2095-8137.2016.5.263
York, J.R. and McCauley, D.W., Functional genetic analysis in a jawless vertebrate, the sea lamprey: insights into the developmental evolution of early vertebrates, J. Exp. Biol., 2020, vol. 223. Suppl. 1, art. ID jeb206433. https://doi.org/10.1242/jeb.206433
York, J.R., Lee, E.M.J., and McCauley, D.W. The Lamprey as a Model Vertebrate in Evolutionary Developmental Biology, in Lampreys: Biology, Conservation and Control. Fish and Fisheries Series, Docker, M., Ed., Dordrecht: Springer, 2019, vol. 38, pp. 481–526 https://doi.org/10.1007/978-94-024-1684-8_6
Zaraisky, A.G., Lukyanov, S.A., Vasiliev, O.L., et al., A novel homeobox gene expressed in the anterior neural plate of the Xenopus embryo, Devel. Biol., 1992, vol. 152, no. 2, pp. 373–382. https://doi.org/10.1016/0012-1606(92)90144-6
Zu, Y., Zhang, X., Ren, J., et al., Biallelic editing of a lamprey genome using the CRISPR/Cas9 system, Sci. Rep., 2016, vol. 6, no. 1, Article 23496. https://doi.org/10.1038/srep23496
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interests.
Statement of the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by O. Zhiryakova
Rights and permissions
About this article
Cite this article
Bayramov, A.V., Ermakova, G.V., Kucheryavyy, A.V. et al. Lamprey as Laboratory Model for Study of Molecular Bases of Ontogenesis and Evolutionary History of Vertebrata. J. Ichthyol. 62, 1213–1229 (2022). https://doi.org/10.1134/S0032945222060029
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945222060029