Abstract
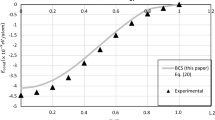
The pairwise effective interionic interaction, the electrical conductivity and thermal EMF coefficients, and the temperature coefficient of resistivity of liquid metallic hydrogen have been calculated in a wide range of densities and temperatures. This range includes both the values achieved in the experiment and those that exist in the central regions of the giant planets. The perturbation theory in the electron–proton interaction potential is used for this. The electrical resistivity is calculated up to the third-order terms of the perturbation theory. Their role proved to be significant. For the ionic subsystem, a hard-sphere model was used. The packing density is considered one of the adjustable parameters of the theory. It is obtained as a function of density and temperature from the analysis of the effective proton–proton interaction. Its adjustment was carried out at the point of transition of hydrogen to the metallic state. Due to the lack of relevant data for metallic hydrogen, the packing density was taken in the same way as for other alkali metals: lithium, sodium, and potassium at their melting point, obtained from neutron scattering experiments.




Similar content being viewed by others
REFERENCES
V. L. Ginzburg, “What problems of physics and astrophysics seem are now to be especially important and interesting (thirty years later, already on the verge of XXI century),” Phys.–Usp. 42, 353–373 (1999).
E. Wigner and N. V. Huntington, “On the possibility of a metallic modification of hydrogen,” J. Chem. Phys. 3, 764–770 (1935).
P. Dias Ranga and F. Silvera Isaac, “Observation of the Wigner–Huntington transition to metallic hydrogen,” Science 355, 715–719 (2017).
S. Deemyad and I. F. Silvera, “The melting line of hydrogen at high pressures,” Phys. Rev. Lett. 100, 155701 (2008).
S. T. Weir, A. C. Mitchell, and W. J. Nellis, “Metallization of fluid molecular hydrogen at 140 GPa (1.4 Mbar),” Phys. Rev. Lett. 76, 1860–1863 (1996).
W. J. Nellis, S. T. Weir, and A. C. Mitchell, “Minimum metallic conductivity of fluid hydrogen at 140 GPa (1.4 Mbar),” Phys. Rev. B 59, 3434–3449 (1999).
W. J. Nellis, “Metastable solid metallic hydrogen,” Philos. Mag. B 79, 655–671 (1999).
M. Bastea, A. C. Mitchell, and W. J. Nellis, “High pressure insulator-metal transition in molecular fluid oxygen,” Phys. Rev. Lett. 86, 3108–3112 (2001).
R. Chau, A. C. Mitchell, R. W. Minich, and W. J. Nellis, “Metallization of fluid nitrogen and the Mott transition in highly compressed low-Z fluids,” Phys. Rev. Lett. 90, 245501(4).
V. E. Fortov, V. Ya. Ternovoi, S. V. Kvitov, V. B. Mintsev, D. N. Nikolaev, A. A. Pyalling, and A. S. Filimonov, “Conductivity of non-ideal hydrogen plasma in the megabar range of dynamic pressures,” Pis’ma Zh. Eksp. Teor. Fiz. 69, 874–878 (1999).
V. Ya. Ternovoy, F. S. Filimonov, V. E. Fortov, S. V. Kvitov, D. N. Nikolaev, and A. A. Pyaling, “Thermodynamic properties and electrical conductivity of hydrogen under multiple shock compression to 150 GPa,” Physica B 265, 6–11 (1999).
J. M. McMahon, M. A. Morales, C. Pierleoni, and D. M. Ceperley, “The properties of hydrogen and helium under extreme conditions,” Rev. Mod. Phys. 84, 1607–1653 (2012).
J. McMinis, R. C. Clay, D. Lee, and M. A. Morales, “Molecular to atomic phase transition in hydrogen under high pressure,” Phys. Rev. Lett. 114, 105305 (2015).
S. Azadi, B. Monserrat, W. M. C. Foulkes, and R. J. Needs, “Dissociation of high-pressure solid molecular hydrogen: A quantum Monte Carlo and anharmonic vibrational study,” Phys. Rev. Lett. 112, 165501 (2014).
V. T. Shvets, “High temperature equation of state of metallic hydrogen,” J. Exp. Theor. Phys. 104, 655–660 (2007).
V. T. Shvets, S. V. Datsko, and Ye. K. Malinovskij, “Metallization degree of hydrogen at a pressure of 1.4 Mbar and a temperature a 3000 K,” Ukr. J. Phys. 52, 70–76 (2007).
J. Waseda and K. Suzuki, “Characteristics of soft core in pour potential and static structure in liquid metals,” Sci. Rep. Res. Inst., Tokio Univ. A 24, 139–184 (1973).
E. G. Brovman, Yu. Kholas, and A. Kagan, “On the structure of metallic hydrogen at zero pressure,” Zh. Eksp. Teor. Fiz. 61, 1300–1315 (1972).
E. G. Brovman, Yu. Kagan, and A. Kholas, “Properties of metallic hydrogen under pressure,” Sov. Phys. JETF 35, 783–787 (1972).
W. A. Harrison, Pseudopotentials in Theory of Metals (W. A. Benjamin, 1966; Mir, Moscow, 1968).
V. T. Shvets, Method of Green Functions in Theory of Metals (Latstar, Odessa, 2002).
V. T. Shvets, Extreme State of Matter. Metallization of Gases (Grin’, Kherson) (in Ukrainian).
T. V. Shvets and V. T. Shvets, “Higher-order perturbation-theory effects in the resistance of simple disordered metals,” Phys. Met. Metallogr. 111, 339–354 (2011).
E. G. Brovman and Yu. Kagan, “Phonons in non-transition metals,” Usp. Fiz. Nauk 112, 369–426 (1974).
D. J. M. Geldart and S. H. Vosko, “The screening function of an interacting electron gas,” Can. J. Phys. 44, 2137–2171 (1966).
L. Ballentine and V. Heine, “On the theory of liquid metals,” Philos. Mag. 9, 617–622 (1964).
V. T. Shvets, S. V. Savenko, and Ye. K. Malinovskii, “Ionic interaction and conductivity of metallic hydrogen,” Condens. Matter Phys. 9, 127–133 (2006).
V. T. Shvets, “Effective proton-proton interaction and metallization of hydrogen,” JETP Lett. 95, 29–32 (2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by E. Chernokozhin
Rights and permissions
About this article
Cite this article
Shvets, V.T. Hydrogen as an Alkaline Metal. Electron Transport Phenomena. Phys. Metals Metallogr. 121, 1–6 (2020). https://doi.org/10.1134/S0031918X20010159
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X20010159