Abstract
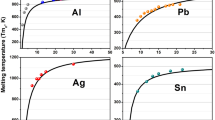
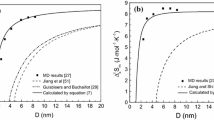
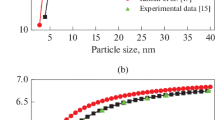
It is necessary to theoretically evaluate the thermodynamic properties of metallic nanoparticles due to the lack of experimental data. Considering the surface effects and crystal structures, a simple theoretical model is developed to study the size dependence of thermodynamic properties of spherical metallic nanoparticles. Based on the model, we have considered Co and Cu nanoparticles for the study of size dependence of cohesive energy, Au and Cu nanoparticles for size dependence of melting temperature, and Cu, Co and Au nanoparticles for size dependence of Debye temperature, respectively. The results show that the size effects on melting temperature, cohesive energy and Debye temperature of the spherical metallic nanoparticles are predominant in the sizes ranging from about 3 nm to 20 nm. The present theoretical predictions are in agreement with available corresponding experimental and computer simulation results for the spherical metallic nanoparticles. The model could be used to determine the thermodynamic properties of other metallic nanoparticles to some extent.
Similar content being viewed by others
References
Y. Y. Gafner, S. L. Gafner, I. S. Zamulin, L. V. Redel and V. S. Baidyshev, “Analysis of the heat capacity of nanoclusters of fcc metals on the example of Al, Ni, Cu, Pd, and Au,” Phys. Met. Metallogr. 116, 568–575 (2015).
Y. D. Qu, X. J. Li, R. Y. Li, H. H. Yan, X. Ouyang and X. H. Wang, “Preparation and characterization of the TiO2 ultrafine particles by detonation method,” Mater. Res. Bull. 43, 97–103 (2008).
Yu. G. Krasnoperova, M. V. Degtyarev, L. M. Voronova, and T. I. Chashchukhina, “Effect of annealing temperature on the recrystallization of nickel with different ultradisperse structures,” Phys. Met. Metallogr. 117, 267–274 (2016).
A. Gohier, C. P. Ewels, T. M. Minea and M. A. Djouadi, “Carbon nanotube growth mechanism switches from tip-to base-growth with decreasing catalyst particle size,” Carbon 46, 1331–1338 (2008).
W. Wang, Y. Zhong, K. Lu, L. Lu, D. L. McDowell and T. Zhu, “Size effects and strength fluctuation in nanoscale plasticity,” Acta Mater. 60, 3302–3309 (2012).
M. A. Kibria, M. R. Anisur, M. H. Mahfuz, R. Saidur and I. H. S. C. Metselaar, “A review on thermophysical properties of nanoparticle dispersed phase change materials,” Energ. Convers. Manag. 95, 69–89 (2015).
L. Gao, H. Z. Wang, J. S. Hong, H. Miyamoto, K. Miyamoto, Y. Nishikawa and S. D. D. L Torre, “Mechanical properties and microstructure of nano-SiC-Al2O3 composites densified by spark plasma sintering,” J. Eur. Ceram. Soc. 19, 609–613 (1999).
R. Z. Valiev, M. Yu. Murashkin, A. V. Ganeev and N. A. Enikeev, “Superstrength of nanostructured metals and alloys produced by severe plastic deformation,” Phys. Met. Metallogr. 113, 1193–1201 (2012)
Y. D. Qu, C. H. Sun, G. L. Sun, X. Q. Kong, and W. J. Zhang, “Preparation, characterization, and kinetic and thermodynamic studies of mixed-phase TiO2 nanoparticles prepared by detonation method,” Res. Phys. 6, 100–106 (2016).
Y. D. Qu, X. J. Li, X. H. Wang and D. H. Liu, “Detonation synthesis of nanosized titanium dioxide powders,” Nanotechnology 18, 205602 (2007).
X. H. Yu and Z. L. Zhan, “The effects of the size of nanocrystalline materials on their thermodynamic and mechanical properties,” Nanoscale Res. Lett. 9, 516–522 (2014).
Y. D. Qu, X. Q. Kong, X. J. Li and H. H. Yan, “Effect of thermal treatment on the structural phase transformation of the detonation-prepared TiO2 mixed crystal nanoparticles,” Acta Phys. Sin. 63, 37301 (2014).
H. K. Kim, S. H. Huh, J.W. Park, J. W. Jeong and G. H. Lee, “The cluster size dependence of thermal stabilities of both molybdenum and tungsten nanoclusters,” Chem. Phys. Lett. 354, 165–172 (2002).
Y. F. Zhu, W. T. Zheng and Q. Jiang, “Modeling lattice expansion and cohesive energy of nanostructured materials,” Appl. Phys. Lett. 95, 083110 (2009).
R. Kumar and M. Kumar, “Effect of size on cohesive energy, melting temperature and Debye temperature of nanomaterials,” Indian J. Pure Appl. Phys. 50, 329–334 (2012).
W. H. Qi and M. P. Wang, “Size effect on the cohesive energy of nanoparticle,” J. Mater. Sci. Lett. 21, 1743–1745 (2002).
G. Guisbiers, “Size-dependent materials properties toward a universal equation,” Nanoscale Res. Lett. 5, 1132–1136 (2010).
L. H. Allen, G. Ramanath, S. L. Lai, Z. Ma, S. Lee, D. D. J. Allman and K. P. Fuchs, “1000000°C/s thin film electrical heater: In situ resistivity measurements of Al and Ti/Si thin films during ultra rapid thermal annealing,” Appl. Phys. Lett. 64, 417–419 (1994).
J. A. Reisland, The Physics of Phonons (Wiley, London, 1973).
C. Kittel, Introduction to Solid State Physics (Wiley, London, 2004).
F. Taherkhani, H. Akbarzadeh, H. Abroshan and A. Fortunelli, “Dependence of self-diffusion coefficient, surface energy, on size, temperature, and Debye temperature on size for aluminum nanoclusters,” Fluid Phase Equilib. 335, 26–31(2012).
M. A. Shandiz, “Effective coordination number model for the size dependency of physical properties of nanocrystals,” J. Phys.: Condens. Matter 20, 325237 (2008).
L. H. Liang, C. M. Shen, S. X. Du, W. M. Liu, X. C. Xie and H. J. Gao, “Increase in thermal stability induced by organic coatings on nanoparticles,” Phys. Rev. B: Condens. Matter Mater. Phys. 70, 205419 (2004).
C. C. Yang and S. Li, “Investigation of cohesive energy effects on size-dependent physical and chemical properties of nanocrystals,” Phys. Rev. B: Condens. Matter Mater. Phys. 75, 165413 (2007).
L. H. Liang, and L. Baowen, “Size-dependent thermal conductivity of nanoscale semiconducting systems,” Phys. Rev. B: Condens. Matter Mater. Phys. 73, 153303 (2006).
G. Guisbiers and S. Pereira, “Theoretical investigation of size and shape effects on the melting temperature of ZnO nanostructures,” Nanotechnology 18, 435710 (2007).
M. Kiguchi, T. Yokoyama, D. Matsummura, H. Kondoh, O. Endo and T. Ohta, “Surface structures and thermal vibrations of Ni and Cu thin films studied by extended X-ray-absorption fine structure,” Phys. Rev. B: Condens. Matter Mater. Phys. 61, 14020–14027 (2000).
C. C. Yang, M. X. Xiao, W. Li and Q. Jiang, “Size effects on Debye temperature, Einstein temperature, and volume thermal expansion coefficient of nanocrystals,” Solid State Commun. 139, 148–152 (2006).
P. Buffat and J. P. Borel, “Size effect of the melting temperature of gold particles,” Phys. Rev. A 13, 2287–2298 (1976).
Y. F. Zhu, W. T. Zheng and Q. Jiang, “Modeling lattice expansion and cohesive energy of nanostructured materials,” Appl. Phys. Lett. 95, 083110 (2009).
M. Cottie, The Weird World of Nanoscale (Univ. Technol., Sydney, Australia, 2007).
L. Wang, Y. Zhang, X. Bian and Y. Chen, “Melting of Cu nanoclusters by molecular dynamics simulation,” Phys. Lett. A 310, 197–202 (2003).
F. Delogu, “Structural and energetic properties of unsupported Cu nanoparticles from room temperature to the melting point: Molecular dynamics simulations,” Phys. Rev. B: Condens. Matter Mater. Phys. 72, 205418 (2005).
K. K. Nanda, S. N. Sahu and S. N. Behera, “Liquiddrop model for the size-dependent melting of lowdimensional systems,” Phys. Rev. A 66, 013208 (2002).
C. C. Yang and S. Li, “Size-, dimensionality-, and composition-dependent Debye temperature of monometallic and bimetallic nanocrystals in the deep nanometer scale,” Phys. Status Solidi B 248, 1375–1378(2011).
H. Yildirim, A. Kara and T. S. Rahman, “Structural, vibrational and thermodynamic properties of Ag n Cu(34–n) nanoparticles” J. Phys.: Condens. Matter, 21, 084220 (2009).
M. Hou, M. E. Azzaoui and H. Pattyn, J. Verheyden, G. Koops and G. Zhang, “Growth and lattice dynamics of Co nanoparticles embedded in Ag: A combined molecular-dynamics simulation and Mössbauer study,” Phys. Rev. B: Condens. Matter Mater. Phys. 62, 5117–5128 (2000).
S. Y. Xiong, W. H. Qi, Y. J. Cheng, B. Y. Huang, M. P. Wang and Y. J. Li, “Universal relation for size dependent thermodynamic properties of metallic nanoparticles,” Phys. Chem. Chem. Phys., 13, 10652–10660 (2011).
A. Balerna, E. Bernieri, P. Picozzi, A. Reale, S. Santucci, E. Burattini and S. Mobilio, “Extended X-rayabsorption fine-structure and near-edge-structure studies on evaporated small clusters of Au,” Phys. Rev. B: Condens. Matter 31, 5058–5065 (1985).
K. Sadaiyandi, “Size dependent Debye temperature and mean square displacements of nanocrystalline Au, Ag and Al,” Mater. Chem. Phys., 115, 703–706 (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Qu, Y.D., Liang, X.L., Kong, X.Q. et al. Size-dependent cohesive energy, melting temperature, and Debye temperature of spherical metallic nanoparticles. Phys. Metals Metallogr. 118, 528–534 (2017). https://doi.org/10.1134/S0031918X17060102
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0031918X17060102