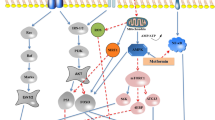
Abstract
Well-known theories of aging suggest that a certain metabolic defect negatively affects vital activity of the cell, be it oxidative stress, the accumulation of lesions in DNA, the exhaustion of telomeres, or distorted epigenetic processes. The theory of aging considered in the review postulates that an accumulation of progerin on the inner side of the nuclear envelope underlies the above defects. Progerin is a defective precursor of the lamin A nuclear matrix protein in which the C-terminal cysteine, which is removed normally, is retained and modified with a hydrophobic oligoisoprene chain. Progerin molecules attach with their hydrophobic processes to the inner membrane of the nuclear envelope, pushing away the adjacent fibrils of the nuclear matrix and the chromatin periphery. This changes the morphology and shape of the nucleus and alters the properties of the nuclear envelope and pore complexes embedded in it. As progerin accumulates in the nucleus, structural distortions increase in the nucleus, further distorting the nuclear–cytoplasmic transport of macromolecules and leading to the above defects in cell metabolism. This leads to increasing cell death and aging of the body over time. This mechanism of aging has been identified in patients with Hutchinson–Gilford progeria syndrome (HGPS). Mass progerin production in HGPS is caused by the point mutation c.1824C→T in exon 11 of the LMNA gene, which codes for lamins A and C. The mutation stimulates nonstandard splicing of the primary transcript during the formation of the lamin A precursor mRNA, thus causing progerin production. Children with progeria who have received the mutation from one of their parents age rapidly and die before 15 years of age. Approaches to progeria treatment are aimed at preventing the formation of progerin or destroying the progerin that has already accumulated. In the latter case, a promising strategy is to use rapamycin or its analogs and other substances and techniques that activate autophagy to purify the cell from progerin. Although in much smaller amounts, progerin is found in progeria-free people and may therefore play a role in natural aging. A maximum age that a person can reach is possible to estimate by taking account of the role that progerin plays in telomere shortening. Encouraging preliminary results achieved in purifying cells from progerin provide a means to develop an optimal procedure for periodic purification of the human body from progerin in order to reduce the rate of aging.






Similar content being viewed by others
Notes
The regularity is inapplicable to between-species comparisons. For example, mouse telomeres are initially twice as long as human telomeres, while the lifespan is many times shorter in mice. The shortening rate was identified as a parameter to be compared between species. The lower the telomere shortening rate, the longer is the lifespan [160, 180].
REFERENCES
Mosevitskii M.I. 2018. Rasprostranennost’ zhizni i unikal’nost’ razuma (The Wide Spread of Life and Uniqueness of Mind). St. Petersburg: SpetsLit.
van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. 2019. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 21, 425–435. https://doi.org/10.1002/ejhf.1320
Proshkina E.N., Solov’eva I.A., Shaposhnikova M.V., Moskaleva A.A. 2020. Key molecular mechanisms of aging, biomarkers, and potential interventions. Mol. Biol. (Moscow). 54 (6), 777–811.
Romano A.D., Serviddio G., de Matthaeis A., Bellanti F., Vendemiale G. 2010. Oxidative stress and aging. J. Nephrol. 23 (Suppl 15), S29–536.
Skulachev V.P., Shilovsky G.A., Putyatina T.S., Popov N.A., Markov A.V., Skulachev M.V., Sadovnichii V.A. 2020. Perspectives of Homo sapiens lifespan extension: Focus on external or internal resources? Aging (Albany, NY). 12, 5566–5584. https://doi.org/10.18632/aging.102981
Best B.P. 2009). Nuclear DNA damage as a direct cause of aging. Rejuvenation Res. 12, 199–208.https://doi.org/10.1089/rej.2009.0847
Olovnikov A.M. 1973. A theory of merginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J. Theor. Biol. 41, 181–190. https://doi.org/10.1016/0022-5193(73)90198-7
Mikhelson V.M., Gamaleya I.A. 2013. Telomeirc Theory of Aging: A Review. Saarbrücken: Palmarium Acad. Publ.
Snow C.J., Dar A., Dutta A., Kehlenbach R.H., Paschal B.M. 2013. Defective nuclear import of TPR in progeria reflects the ran sensitivity of large cargo transport. J. Cell Biol. 201, 541–557. https://doi.org/10.1083/jcb.201212117
Fasci D., van Ingen H., Scheltema R.A., Heck A.J.R. 2018. Histone interaction landscapes visualized by crosslinking mass spectrometry in intact cell nuclei. Mol. Cell. Proteomics. 17, 2018–2033. https://doi.org/10.1074/mcp.RA118.000924
Dworak N., Makosa D., Chatterjee M., Jividen K., Yang C.S., Snow C., Simke W.C., Johnson I.G., Kelley J.B., Paschal B.M. 2019. A nuclear lamina-chromatin-Ran GTPase axis modulates nuclear import and DNA damage signaling. Aging Cell. 18, e12851. https://doi.org/10.1111/acel
Güttler T., Görlich D. 2011. Ran-dependent nuclear export mediators: A structural perspective. EMBO J. 30, 3457–3474. https://doi.org/10.1038/emboj.2011.287
Goldberg M.W., Huttenlauch I., Hutchison C.J., Stick R. 2008. Filaments made from A- and B-type lamins differ in structure and organization. J. Cell Sci. 121, 215–225. https://doi.org/10.1242/jcs.022020
Zbarsky I.B., Georgiev G.P. 1959. Cytological characteristics of protein and nucleoprotein fractions of cell nuclei. Biochim. Biophys. Acta. 32 (1), 301–302. https://doi.org/10.1016/0006-3002(59)90600-6
Georgiev G.P., Chentsov Yu.S.1963. On ultrastructure of the nucleus: Basic structural elements of cell nuclei and their nucleoprotein composition. Biofizika. 8, 50–57.
Earnshaw W.C., Laemmli U.K. 1983. Architecture of metaphase chromosomes and chromosome scaffolds. J. Cell Biol. 96, 84–93. https://doi.org/10.1083/jcb.96.1.84
Smith H.C., Puvion E., Buchholtz L.A., Berezney R. 1984. Spatial distribution of DNA loop attachment and replicational sites in the nuclear matrix. J. Cell Biol. 99, 1794–1802. https://doi.org/10.1083/jcb.99.5.1794
Mortillaro M.J., Blencowe B.J., Wei X., Nakayasu H., Du L., Warren S.L., Sharp P.A., Berezney R. 1996. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc. Natl. Acad. Sci. U. S. A. 93, 8253–8257. https://doi.org/10.1073/pnas.93.16.8253
Wei X., Somanathan S., Samarabandu J., Berezney R. 1999. Thre0e-dimensional visualization of transcription sites and their association with splicing factor-rich nuclear speckles. J. Cell Biol. 146, 543–558.
Razin S.V., Iarovaia O.V., Y.S. Vassetzky Y.S. 2014. A requiem to the nuclear matrix: From a controversial concept to 3D organization of the nucleus. Chromosoma. 123, 217–224. https://doi.org/10.1007/s00412-014-0459-8
Valter S.N., Kachurin A.L., Popov Yu.V., Mosevitsky M.I. 1984. Observation of the intranuclear scaffold formed by a structured fibril network in thin liver sections. Dokl. Akad. Nauk SSSR. 279, 1249–1251.
Adolph K.W. 1980. Organization of chromosomes in HeLa cells: Isolation of histone-depleted nuclei and nuclear scaffolds. J. Cell Sci. 42, 291–304.
Fey E.G., Krochmalnic G., Penman S. 1986. The nonchromatin substructures of the nucleus: The ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J. Cell Biol. 102, 1654–1665. https://doi.org/10.1083/jcb.102.5.1654
Gerace L., Blobel G. 1980. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 19 (1), 277–287. https://doi.org/10.1016/0092-8674(80)90409-2
Turgay Y., Eibauer M., Goldman A.E., Shimi T., Khayat M., Ben-Harush K., Dubrovsky-Gaupp A., Sapra K.T., Goldman R.D., Medalia O. 2017. The molecular architecture of lamins in somatic cells. Nature. 543, 261–264. https://doi.org/10.1038/nature21382
Ahn J., Jo I., Kang S.M., Hong S., Kim S., Jeong S., Kim Y.H., Park B.J., Ha N.C. 2019). Structural basis for lamin assembly at the molecular level. Nat. Commun. 10 (1), 3757. https://doi.org/10.1038/s41467-019-11684-x
Lin F., Worman H.J. 1993. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 268, 16321–16326.
Stroud M.J., Banerjee I., Veevers J., Chen J. 2014. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ. Res. 114, 538–548. https://doi.org/10.1161/CIRCRESAHA.114.301236
Stroud M.J. 2018. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiomyopathy. Biophys. Rev. 10, 1033–1051. https://doi.org/10.1007/s12551-018-0431-6
Worman H.J., Yuan J., Blobel G., Georgatos S.D. 1988. A lamin B receptor in the nuclear envelope. Proc. Natl. Acad. Sci. U. S. A. 85, 8531–8534. https://doi.org/10.1073/pnas.85.22.8531
Smith S., Blobel G. 1993. The first membrane spanning region of the lamin B receptor is efficient for sorting to the inner nuclear membrane. J. Cell Biol. 120, 631–637.
Olins A.L., Rhodes G., Welch D.B., Zwerger M., Olins D.E. 2010. Lamin B receptor: Multi-tasking at the nuclear envelope. Nucleus. 1, 53–70. https://doi.org/10.4161/nucl.1.1.10515
Liokatis S., Edlich C., Soupsana K., Giannios I., Panagiotidou P., Tripsianes K., Sattler M., Georgatos S.D., Politou A.S. 2012. Solution structure and molecular interactions of lamin B receptor tudor domain. J. Biol. Chem. 287, 1032–1042. https://doi.org/10.1074/jbc.M111.281303
Nikolakaki E., Mylonis I., Giannakouros T. 2017. Lamin B receptor: Interplay between structure, function and localization. Cells. 6, 28. https://doi.org/10.3390/cells6030028
Constantinescu D., Gray H.L., Sammak P.J., Schatten G.P., Csoka A.B. 2006. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 24, 177–185. https://doi.org/10.1634/stemcells.2004-0159
Gruenbaum Y., Foisner R. 2015. Lamins: Nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation Annu. Rev. Biochem. 84, 131–164. https://doi.org/10.1146/annurev-biochem-060614-034115
Zhang H., Petrie M.V., He Y., Peace J.M., Chiolo I.E., Aparicio O.M. 2019. Dynamic relocalization of replication origins by Fkh1 requires execution of DDK function and Cdc45 loading at origins. eLife. 8, e45512. https://doi.org/10.7554/eLife.45512
Bermeo S., Vidal C., Zhou H., Duque G. 2015. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/β-catenin pathway. J. Cell Biochem. 116, 2344–2353.
Davidson K.C., Adams A.M., Goodson J.M., McDonald C.E., Potter J.C., Berndt J.D., Biechele T.L., Taylor R.J., Moon R.T. 2012. Wnt/β-catenin signaling promotes differentiation, not self-renewal, of human embryonic stem cells and is repressed by Oct4. Proc. Natl. Acad. Sci. U. S. A. 109, 4485–4490.
Duque G., Rivas D. 2006. Age-related changes in lamin A/C expression in the osteoarticular system: Laminopathies as a potential new aging mechanism. Mech. Ageing Dev. 127, 378–383. https://doi.org/10.1016/j.mad.2005.12.007
Forleo C., Carmosino M., Resta N., Rampazzo A., Valecce R., Sorrentino S., Iacoviello M., Pisani F., Procino G., Gerbino A., Scardapane A., Simone C., Calore M., Torretta S., Svelto M., Favale S. 2015. Clinical and functional characterization of a novel mutation in lamin A/C gene in a multigenerational family with arrhythmogenic cardiac laminopathy. PLoS One. 10 (4), e0121723. https://doi.org/10.1371/journal.pone.0121723
Crasto S., My I., Pasquale E.D. 2020. The broad spectrum of LMNA cardiac diseases: From olecular mechanisms to clinical phenotype. Front. Physiol. 11, 761. https://doi.org/10.3389/fphys.2020.00761
Pollex R.L., Hegele R.A. 2004. Hutchinson–Gilford progeria syndrome. Clin. Genet. 66, 375–381. https://doi.org/10.1111/j.1399-0004.2004.00315.x
Scaffidi P., Misteli T. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312 (5776), 1059–1063. https://doi.org/10.1126/science.1127168
Merideth M.A., Gordon L.B., Clauss S., Sachdev V., Smith A.C., Perry M.B., Brewer C.C., Zalewski C., Kim H.J., Solomon B., Brooks B.P., Gerber L.H., Turner M.L., Domingo D.L., Hart T.C., Graf J., et al. 2008. Phenotype and course of Hutchinson–Gilford progeria syndrome. N. Engl. J. Med. 358, 592–604. https://doi.org/10.1056/NEJMoa0706898
Coutinho H.D.M., Falcão-Silva V.S., Gregório Fernandes Gonçalves G.F., da Nóbrega R.B. 2009. Molecular ageing in progeroid syndromes: Hutchinson–Gilford progeria syndrome as a model. Immun. Ageing. 20, 6–14. https://doi.org/10.1186/1742-4933-6-4
Eriksson M., Brown W.T., Gordon L.B., Glynn M.W., Singer J., Scott L., Erdos M.R., Robbins C.M., Moses T.Y., Berglund P., Dutra A., Pak E., Durkin S., Csoka A.B., Boehnke M., et al. 2003. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 423 (6937), 293–298. https://doi.org/10.1038/nature01629
De Sandre-Giovannoli A., Bernard R., Cau P., Navarro C., Amiel J., Boccaccio I., Lyonnet S., Stewart C.L., Munnich A., Le Merrer M., Lévy N. 2003. Lamin A truncation in Hutchinson–Gilford progeria. Science. 300 (5628), 2055. https://doi.org/10.1126/science.1084125
Capell B.C., Erdos M.R., Madigan J.P., Fiordalisi J.J., Varga R., Conneely K.N., Gordon L.B., Der C.J., Cox A.D., Collins F.S. 2005. Inhibiting farnesylation of progerin prevents the characteristic nuclear blebbing of Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 102, 12879–12884. https://doi.org/10.1073/pnas.0506001102
Glynn M.W., Glover T.W. 2005. Incomplete processing of mutant lamin A in Hutchinson–Gilford progeria leads to nuclear abnormalities, which are reversed by farnesyltransferase inhibition. Hum. Mol. Genet. 14, 2959–2969. https://doi.org/10.1093/hmg/ddi326
Cenni V., Capanni C., Mattioli E., Schena E., Squarzoni S., Bacalini M.G., Garagnani P., Salvioli S., Franceschi C., Lattanzi G. 2020. Lamin A involvement in ageing processes. Ageing Res. Rev. 62, 101073. https://doi.org/10.1016/j.arr.2020.101073
Chojnowski A., Ong P.F., Wong E.S., Lim J.S., Mutalif R.A., Navasankari R., Dutta B., Yang H., Liow Y.Y., Sze S.K., Boudier T., Wright G.D., Colman A., Burke B., Stewart C.L., Dreesen O. 2015. Progerin reduces LAP2α-telomere association in Hutchinson–Gilford progeria. eLife. 4, e07759. https://doi.org/10.7554/eLife.07759
Chojnowski A., Ong P.F., Wong E.S., Lim J.S., Mutalif R.A., Navasankari R., Dutta B., Yang H., Liow Y.Y., Sze S.K., Boudier T., Wright G.D, Colman A., Burke B., Stewart C.L., Dreesen O. 2020. Heterochromatin loss as a determinant of progerin-induced DNA damage in Hutchinson–Gilford progeria. Aging Cell. 19, e13108. https://doi.org/10.1111/acel.13108
Romero-Bueno R., de la Cruz Ruiz P., Artal-Sanz M., Askjaer P., Dobrzynska A. 2019. Nuclear organization in stress and aging. Cells. 8, 664. https://doi.org/10.3390/cells8070664
Martins F., Sousa J., Pereira C.D., da Cruz e Silva O.A.B., Rebelo S. 2020. Nuclear envelope dysfunction and its contribution to the aging process. Aging Cell. 19, e13143. https://doi.org/10.1111/acel.13143
Arii J., Maeda F., Maruzuru Y., Koyanagi N., Kato A., Mori Y., Kawaguchi Y. 2020. ESCRT-III controls nuclear envelope deformation induced by progerin. Sci. Rep. 10, 18877. https://doi.org/10.1038/s41598-020-75852-6
Kang S.M., Yoon M.H., Ahn J., Kim J.E., Kim S.Y., Kang S.Y., Joo J., Park S., Cho J.H., Woo T.G., Oh A.Y., Chung K.J., An S.Y., Hwang T.S., Lee S.Y., et al. 2021. Progerinin, an optimized progerin-lamin A binding inhibitor, ameliorates premature senescence phenotypes of Hutchinson–Gilford progeria syndrome. Commun. Biol. 4, 5. https://doi.org/10.1038/s42003-020-01540-w
Goldman R.D., Shumaker D.K., Erdos M.R., Eriksson M., Goldman A.E., Gordon L.B., Gruenbaum Y., Khuon S., Mendez M., Varga R., Collins F.S. 2004. accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 101, 8963–8968. https://doi.org/10.1073/pnas.0402943101
Dahl K.N., Scaffidi P., Islam M.F., Yodh A.G., Wilson K.L., Misteli T. 2006. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 103, 10271–10276.
Cao K., Graziotto J.J., Blair C.D., Mazzulli J.R., Erdos M.R., Krainc D., Collins F.S. 2011. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson–Gilford progeria syndrome. Cells. Sci. Transl. Med. 3, 89ra58. https://doi.org/10.1126/scitranslmed.3002346
Noda A., Mishima S., Hirai Y., Hamasaki K., Landes R.D., Mitani H., Haga K., Kiyono T., Nakamura N., Kodama Y. 2015. Progerin, the protein responsible for the Hutchinson–Gilford progeria syndrome, increases the unrepaired DNA damages following exposure to ionizing radiation. Genes Environ. 37, 13. https://doi.org/10.1186/s41021-015-0018-4
Saxena S., Kumar S. 2020. pharmacotherapy to gene editing: Potential therapeutic approaches for Hutchinson–Gilford progeria syndrome. Geroscience. 42, 467–494. https://doi.org/10.1007/s11357-020-00167-3
Gabriel D., Roedl D., Gordon L.B., Djabali K. 2015. Sulforaphane enhances progerin clearance in Hutchinson–Gilford progeria fibroblasts. Aging Cell. 14, 78–91. https://doi.org/10.1111/acel.12300
Rivera-Torres J., Acín-Perez R., Cabezas-Sánchez P., Osorio F.G., Gonzalez-Gómez C., Megias D., Cámara C., López-Otín C., Enríquez J.A., Luque-García J.L., Andrés V. 2013. identification of mitochondrial dysfunction in Hutchinson–Gilford progeria syndrome through use of stable isotope labeling with amino acids in cell culture. J. Proteomics. 91, 466–477.
Bidault G., Garcia M., Capeau J., Morichon R., Vigouroux C., Béréziat V. 2020. Progerin expression induces inflammation, oxidative stress and senescence in human coronary endothelial cells. Cells. 9 (5), 1201. https://doi.org/10.3390/cells9051201
Chen W.M., Chiang J.C., Lin Y.C., Lin Y.N., Chuang P.Y., Chang Y.C., Chen C.C., Wu K.Y., Hsieh J.C., Chen S.K., Huang W.P., Chen B.P.C., Lee H. 2020. Lysophosphatidic acid receptor LPA3 prevents oxidative stress and cellular senescence in Hutchinson–Gilford progeria syndrome. Aging Cell. 19, e13064. https://doi.org/10.1111/acel.13064
Mao X., Bharti P., Thaivalappil A., Cao K. 2020. peroxisomal abnormalities and catalase deficiency in Hutchinson–Gilford progeria syndrome. Aging (Albany, NY). 12, 5195–5208. https://doi.org/10.18632/aging.102941
Bandaria J.N., Qin P., Berk V., Chu S., Yildiz A. 2016. Shelterin protects chromosome ends by compacting telomeric chromatin. Cell. 164, 735–746. https://doi.org/10.1016/j.cell.2016.01.036
Prokocimer M., Barkan R., Gruenbaum Y. 2013. Hutchinson–Gilford progeria syndrome through the lens of transcription. Aging Cell. 12, 533–543. https://doi.org/10.1111/acel.12070
Arancio W., Pizzolanti G., Genovese S.I., Pitrone M., Giordano C. 2014. Epigenetic involvement in Hutchinson–Gilford progeria syndrome: A mini-review. Gerontology. 60, 197–203. https://doi.org/10.1159/000357206
Bär C., Blasco M.A. 2016). Telomeres and telomerase as therapeutic targets to prevent and treat age-related diseases. F1000Res. 5, F1000 Faculty Rev-89. https://doi.org/10.12688/f1000research.7020.1
Gavia-García G., Rosado-Pérez J., Arista-Ugalde T.L., Aguiñiga-Sánchez I., Santiago-Osorio E., Mendoza-Núñez V.M. 2021. Telomere length and oxidative stress and its relation with metabolic syndrome components in the aging. Biology (Basel). 10 (4), 253. https://doi.org/10.3390/biology10040253
Schoeftner S., Blasco M.A. 2008. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat. Cell. Bio. 10, 228–236. https://doi.org/10.1038/ncb1685
Redon S., Reichenbach P., Lingner J. 2010. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 38, 5797–5806. https://doi.org/10.1093/nar/gkq296
Jiang X., Wang L., Xie S., Chen Y., Song S., Lu Y., Lu D. 2020. Long noncoding RNA MEG3 blocks telomerase activity in human liver cancer stem cells epigenetically. Stem Cell Res. Ther. 11 (1), 518. https://doi.org/10.1186/s13287-020-02036-4
Pfeiffer V., Lingner J. 2012. TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet. 8 (6), e1002747. https://doi.org/10.1371/journal.pgen.1002747
Huang S., Risques R.A., Martin G.M., Rabinovitch P.S., Oshima J. 2008. Accelerated telomere shortening and replicative senescence in human fibroblasts overexpressing mutant and wild-type lamin A. Exp. Cell. Res. 314 (1), 82–91. https://doi.org/10.1016/j.yexcr.2007.08.004
Aguado J., Sola-Carvajal A., Cancila V., Revêchon G., Ong P.F., Jones-Weinert C.W., Wallén Arzt E., Lattanzi G., Dreesen O., Tripodo C., Rossiello F., Eriksson M., d’Adda di Fagagna F. 2019. Inhibition of DNA damage response at telomeres improves the detrimental phenotypes of Hutchinson–Gilford progeria syndrome. Nat. Commun. 10 (1), 4990. https://doi.org/10.1038/s41467-019-13018-3
Mallampalli M.P., Huyer G., Bendale P., Gelb M.H., Michaelis S. 2005. inhibiting farnesylation reverses the nuclear morphology defect in a HeLa cell model for Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. U. S. A. 102, 14416–14421. https://doi.org/10.1073/pnas.0503712102
Toth J.I., Yang S.H., Qiao X., Beigneux A.P., Gelb M.H., Moulson C.L., Miner J.H., Young S.G., Fong L.G. 2005. Blocking protein farnesyltransferase improves nuclear shape in fibroblasts from humans with progeroid syndromes. Proc. Natl. Acad. Sci. U. S. A. 102, 12873–12878. https://doi.org/10.1073/pnas.0505767102
Yang S.H., Bergo M.O., Toth JI., Qiao X., Hu Y., Sandoval S., Meta M., Bendale P, Gelb M.H., Young S.G., Fong L.G. 2005. blocking protein farnesyltransferase improves nuclear blebbing in mouse fibroblasts with a targeted Hutchinson–Gilford progeria syndrome mutation. Proc. Natl. Acad. Sci. U. S. A. 102, 10291–10296. https://doi.org/10.1073/pnas.0504641102
Yang S.H., Meta M., Qiao X., Frost D., Bauch J., Coffinier C., Majumdar S., Bergo M.O., Young S.G., Fong L.G. 2006. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson–Gilford progeria syndrome mutation. J. Clin. Invest. 116, 2115–2121. https://doi.org/10.1172/JCI28968
Fong L.G., Frost D., Meta M., Qiao X., Yang S.H., Coffinier C., Young S.G. 2006. A protein farnesyltransferase inhibitor ameliorates disease in a mouse model of progeria. Science. 311 (5767), 1621–1623. https://doi.org/10.1126/science.1124875
Wang Y., Panteleyev A.A., Owens D.M., Djabali K., Stewart C.L., Worman H.J. 2008. Epidermal expression of the truncated prelamin A causing Hutchinson–Gilford progeria syndrome: Effects on keratinocytes, hair and skin. Hum. Mol. Genet. 17, 2357–2369. https://doi.org/10.1093/hmg/ddn136
Wang Y., Ostlund C., Worman H.J. 2010. Blocking protein farnesylation improves nuclear shape abnormalities in keratinocytes of mice expressing the prelamin A variant in Hutchinson–Gilford progeria syndrome. Nucleus. 1, 432–439. https://doi.org/10.4161/nucl.1.5.12972
Cubria M.B., Suarez S., Masoudi A., Oftadeh R, Kamalapathy P., DuBose A., Erdos M.R., Cabral W.A., Karim L., Collins F.S., Snyder B.D., Nazarian A. 2020. Evaluation of musculoskeletal phenotype of the G608G progeria mouse model with lonafarnib, pravastatin, and zoledronic acid as treatment groups Proc. Natl. Acad. Sci. U. S. A. 117, 12029–12040. https://doi.org/10.1073/pnas.1906713117
Lai W.F., Wong W.T. 2020. Progress and trends in the development of therapies for Hutchinson–Gilford progeria syndrome. Aging Cell. 19 (7), e13175. https://doi.org/10.1111/acel.13175
Dhillon S. 2021. Lonafarnib: First approval. Drugs. 81, 283–289. https://doi.org/10.1007/s40265-020-01464-z
Blondel S., Egesipe A.L., Picardi P., Jaskowiak A.L., Notarnicola M., Ragot J., Tournois J., Le Corf A., Brinon B., Poydenot P., Georges P., Navarro C., Pitrez P.R., Ferreira L., Bollot G., et al. 2016. Drug screening on Hutchinson–Gilford progeria pluripotent stem cells reveals aminopyrimidines as new modulators of farnesylation. Cell. Death Dis. 7 (2), 2105. https://doi.org/10.1038/cddis.2015.374
Gordon L.B., Shappell H., Massaro J., D’Agostino R.B. Sr., Brazier J., Campbell S.E., Kleinman M.E., Kieran M.W. 2018. association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson–Gilford progeria syndrome. JAMA. 319, 1687–1695. https://doi.org/10.1001/jama.2018.3264
Young S.G., Yang S.H., Davies B.S., Jung H.J., Fong L.G. 2013. Targeting protein prenylation in progeria. Sci. Transl. Med. 5 (171), 171ps3. https://doi.org/10.1126/scitranslmed.3005229
Scaffidi P., Misteli T. 2005). Reversal of the cellular phenotype in the premature aging disease Hutchinson–Gilford progeria syndrome. Nat. Med. 11, 440-445. https://doi.org/10.1038/nm1204
Osorio F.G., Navarro C.L., Cadiñanos J., López-Mejía I.C., Quirós P.M., Bartoli C., Rivera J., Tazi J., Guzmán G., Varela I., Depetris D., de Carlos F., Cobo J., Andrés V., De Sandre-Giovannoli A., et al. 2011. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci. Transl. Med. 3 (106), 106ra107. https://doi.org/10.1126/scitranslmed.3002847
Erdos M.R., Cabral W.A., Tavarez U.L., Cao K., Gvozdenovic-Jeremic J., Narisu N., Zerfas P.M., Crumley S., Boku Y., Hanson G., Mourich D.V., Kole R., Eckhaus M.A., Gordon L.B., Collins F.S. 2021. A targeted antisense therapeutic approach for Hutchinson–Gilford progeria syndrome. Nat. Med. 27, 536–545. https://doi.org/10.1038/s41591-021-01274-0
Puttaraju M., Jackson M., Klein S., Shilo A., Bennett C.F., Gordon L., Rigo F., Misteli T. 2021. Systematic screening identifies therapeutic antisense oligonucleotides for Hutchinson–Gilford progeria syndrome. Nat. Med. 27, 526–535. https://doi.org/10.1038/s41591-021-01262-4
Revêchon G., Whisenant D., Eriksson M. 2021. Splice-inhibition therapy targets progeria. Nat. Med. 27, 377–379. https://doi.org/10.1038/s41591-021-01267-z
Pellegrini C., Columbaro M., Capanni C., D’Apice M.R., Cavallo C., Murdocca M., Lattanzi G., Squarzoni S. 2015. All-trans retinoic acid and rapamycin normalize Hutchinson–Gilford progeria fibroblast phenotype. Oncotarget. 6, 2914–2928. https://doi.org/10.18632/oncotarget.4939
Kreienkamp R., Croke M., Neumann M.A., Bedia-Diaz G., Graziano S., Dusso A., Dorsett D., Carlberg C., Gonzalo S. 2016. Vitamin D receptor signaling improves Hutchinson–Gilford progeria syndrome cellular phenotypes. Oncotarget. 7 (21), 30018–30031. https://doi.org/10.18632/oncotarget.9065
Beyret E., Liao H.K., Yamamoto M., Hernandez-Benitez R., Fu Y., Erikson G., Reddy P., Izpisua Belmonte J. 2019. Single-dose CRISPR-Cas9 therapy extends lifespan of mice with Hutchinson–Gilford progeria syndrome. Nat. Med. 25, 419–422. https://doi.org/10.1038/s41591-019-0343-4
Piekarowicz K., Machowska M.,Volha Dzianisava V., Rzepecki R. 2019. Hutchinson–Gilford progeria syndrome-current status and prospects for gene therapy treatment. Cells. 8 (2), 88. https://doi.org/10.3390/cells8020088
Santiago-Fernández O., Osorio F.G., Quesada V., Rodríguez F., Basso S., Maeso D., Rolas L, Barkaway A., Nourshargh S., Folgueras A.R., Freije J.M.P., López-Otín C. 2019. Development of a CRISPR/Cas9-based therapy for Hutchinson–Gilford progeria syndrome. Nat. Med. 25, 423–426. https://doi.org/10.1038/s41591-018-0338-6
Marraffini L.A., Sontheimer E.J. 2010. CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat. Rev. Genet. 11, 181–190. https://doi.org/10.1038/nrg2749
Wiedenheft B. 2013). In defense of phage: Viral suppressors of CRISPR-mediated adaptive immunity in bacteria. RNA Biol. 10, 886–890. https://doi.org/10.4161/rna.23591
Wu S.-S., Li Q.-C., Yin C.-Q., Xue W., Song C.-Q. 2020. Advances in CRISPR/Cas-based gene therapy in human genetic diseases. Theranostics. 10, 4374–4382. https://doi.org/10.7150/thno.43360
Koblan L.W., Erdos M.R., Wilson C., Cabral W.A., Levy J.M., Xiong Z.M., Tavarez U.L., Davison L.M., Gete Y.G., Mao X., Newby G.A., Doherty S.P., Narisu N., Sheng Q., Krilow C., et al. 2021. In vivo base editing rescues Hutchinson–Gilford progeria syndrome in mice. Nature. 589 (7843), 608–614. https://doi.org/10.1038/s41586-020-03086-7
Gaudelli N.M., Komor A.C., Rees H.A., Packer M.S., Badran A.H., Bryson D.I., Liu D.R. 2017. Programmable base editing of A-T to G-C in genomic DNA without DNA cleavage. Nature. 551 (7681), 464–471. https://doi.org/10.1038/nature24644
Graziotto J.J., Cao K., Collins F.S., Krainc D. 2012. Rapamycin activates autophagy in Hutchinson–Gilford progeria syndrome: Implications for normal aging and age-dependent neurodegenerative disorders. Autophagy. 8, 147–151. https://doi.org/10.4161/auto.8.1.18331
Ehninger D., Neff F, Xie K. 2014. Longevity, aging and rapamycin. Cell. Mol. Life Sci. 71, 4325–4346. https://doi.org/10.1007/s00018-014-1677-1
Mendelsohn A.R., Larrick J.W. 2011. Rapamycin as an antiaging therapeutic?: Targeting mammalian target of rapamycin to treat Hutchinson–Gilford progeria and neurodegenerative diseases. Rejuvenation Res. 14, 437–441. https://doi.org/10.1089/rej.2011.1238
Ramos F.J., Chen S.C., Garelick M.G, Dai D.F., Liao C.Y., Schreiber K.H., MacKay V.L., An E.H., Strong R., Ladiges W.C., Rabinovitch P.S., Kaeberlein M., Kennedy B.K. 2012. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 4 (144), 144ra103. https://doi.org/10.1126/scitranslmed.3003802
Yang H., Rudge D.G., Koos J.D., Vaidialingam B., Yang H.J., Pavletich N.P. 2013. mTOR kinase structure, mechanism and regulation. Nature. 497, 217–223. https://doi.org/10.1038/nature12122
Clements C.S., Bikkul M.U., Ofosu W., Eskiw C., Tree D., Makarov E., Kill I.R., Bridger J.M. 2019. Presence and distribution of progerin in HGPS cells is ameliorated by drugs that impact on the mevalonate and mTOR pathways. Biogerontology. 20, 337–358. https://doi.org/10.1007/s10522-019-09807-4
Papadopoli D., Boulay K., Kazak L., Pollak M., Mallette F.A., Topisirovic I., Hulea L. 2019. mTOR as a central regulator of lifespan and aging. F1000Res. 8, F1000 Faculty Rev-998. https://doi.org/10.12688/f1000research.17196.1
Saxton R.A., Sabatini D.M. 2017. mTOR signaling in growth, metabolism, and disease. Cell. 169, 361–371.
Huang J.U., Klionsky D.J. 2007. Autophagy and human disease. Cell Cycle. 6, 1837–1849. https://doi.org/10.4161/cc.6.15.4511
Kim Y.Ch., Guan K.-L. 2015. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Invest. 125, 25–32. https://doi.org/10.1172/JCI73939
Sotthibundhu A. 2016. Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res. Ther. 7, 166. https://doi.org/10.1186/s13287-016-0425-x
Almendáriz-Palacios C., Gillespie Z.E., Janzen M., Martinez V., Bridger J.M., Harkness T.A.A., Mousseau D.D., Eskiw C.H. 2020. The nuclear lamina: Protein accumulation and disease. Biomedicines. 8 (7), 188. https://doi.org/10.3390/biomedicines8070188
Saegusa C., Hosoya M., Nishiyama T., Saeki T., Fujimoto C., Okano H., Fujioka M., Ogawa K. 2020. Low-dose rapamycin-induced autophagy in cochlear outer sulcus cells. Laryngoscope Investig. Otolaryngol. 5, 520–528. https://doi.org/10.1002/lio2.392
Mizushima N., Levine B.N. 2020. Autophagy in human diseases. N. Engl. J. Med. 383 (16), 1564–1576. https://doi.org/10.1056/NEJMra2022774
Lu X., Djabali K. 2018. Autophagic removal of farnesylated carboxy-terminal lamin peptides. Cells. 7 (4), 33. https://doi.org/10.3390/cells7040033
Cenni V., Capanni C., Columbaro M., Ortolani M., D’Apice M.R., Novelli G., Fini M., Marmiroli S., Scarano E., Maraldi N.M., Squarzoni S., Prencipe S., Lattanzi G. 2011. Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria. Eur. J. Histochem. 55 (4), e36. https://doi.org/10.4081/ejh.2011.e36
Strong R., Miller R.A., Bogue M., Fernandez E., Javors M.A., Libert S., Marinez P.A., Murphy M.P., Musi N., Nelson J.F., Petrascheck M., Reifsnyder P., Richardson A., Salmon A.B., Macchiarini F., Harrison D.E. 2020. Rapamycin-mediated mouse lifespan extension: Late-life dosage regimes with sex-specific effects. Aging Cell. 19 (11), e13269.https://doi.org/10.1111/acel.13269
Zhang Y., Zhang J., Wang S. 2021. The role of rapamycin in healthspan extension via the delay of organ aging. Ageing Res Rev. 70, 101376. https://doi.org/10.1016/j.arr.2021.101376
Garay R.P. 2021. Investigational drugs and nutrients for human longevity. Recent clinical trials registered in ClinicalTrials.gov and clinicaltrialsregister.eu. Expert Opin. Investig. Drugs. 30, 749–758. https://doi.org/10.1080/13543784.2021.1939306
Peters J.M., Franke W.W., Kleinschmidt J.A. 1994. Distinct 19S and 20S subcomplexes of the 26S proteasome and their distribution in the nucleus and the cytoplasm. J. Biol. Chem. 269, 7709–7718.
Sorokin A.V., Kim E.R., Ovchinnikov L.P. 2009. The proteasome system of protein degradation and processing. Usp. Biol. Khim. 49, 3–76.
Harhouri K., Navarro C., Depetris D., Mattei M.G., Nissan X., Cau P., De Sandre-Giovannoli A., Lévy N. 2017. MG132-induced progerin clearance is mediated by autophagy activation and splicing regulation. EMBO Mol. Med. 9, 1294–1313. https://doi.org/10.15252/emmm.201607315
Harhouri K., Frankel D., Bartoli C., Roll P., De Sandre-Giovannoli A., Lévy N. 2018. An overview of treatment strategies for Hutchinson–Gilford progeria syndrome. Ucleus. 9, 246–257. https://doi.org/10.1080/19491034.2018.1460045
McClintock D., Ratner D., Lokuge M., Owens D.M., Gordon L.B., Collins F.S., Djabali K. 2007. The mutant form of lamin A that causes Hutchinson–Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2, e1269. https://doi.org/10.1371/journal.pone.0001269
Rodriguez S., Coppedè F., Sagelius H., Eriksson M. 2009. Increased expression of the Hutchinson–Gilford progeria syndrome truncated lamin a transcript during cell aging. Eur. J. Hum. Genet. 17, 928–937. https://doi.org/10.1038/ejhg.2008.270
Ashapkin V.V., Kutueva L.I., Kurchashova S.Y., Kireev I.I. 2019. Are there common mechanisms between the Hutchinson–Gilford progeria syndrome and natural aging? Front. Genet. 10, 455. https://doi.org/10.3389/fgene.2019.00455
Kreienkamp R., Gonzalo S. 2020. Metabolic dysfunction in Hutchinson–Gilford progeria syndrome. Cells. 9, 395. https://doi.org/10.3390/cells9020395
Osorio F.G., Varela I., Lara E., Puente X.S., Espada J., Santoro R., Freije J.M., Fraga M.F., López-Otín C. 2010. Nuclear envelope alterations generate an aging-like epigenetic pattern in mice deficient in Zmpste24 metalloprotease. Aging Cell. 9, 947–957. https://doi.org/10.1111/j.1474-9726.2010.00621.x
Worman H.J., Michaelis S. 2018. Permanently farnesylated prelamin A, progeria, and atherosclerosis. Circulation. 138, 283–286. https://doi.org/10.1161/CIRCULATIONAHA.118.034480
Kawakami Y., Hambright W.S., Takayama K., Mu X., Lu A., Cummins J.H., Matsumoto T., Yurube T., Kuroda R., Kurosaka M., Fu F.H., Robbins P.D., Niedernhofer L.J., Huard J. 2019. Rapamycin rescues age-related changes in muscle-derived stem/progenitor cells from progeroid mice. Mol. Ther. Methods Clin. Dev. 14, 64–76. https://doi.org/10.1016/j.omtm.2019.05.011
Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., Pahor M., Javors M.A., Fernandez E., Miller R.A. 2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 460 (7253), 392–395. https://doi.org/10.1038/nature08221
Stacchiotti A., Corsetti G. 2020. Natural compounds and autophagy: Allies against neurodegeneration. Front Cell Dev. Biol. 8, 555409. https://doi.org/10.3389/fcell.2020.555409
Yessenkyzy A., Saliev T., Zhanaliyeva M., Masoud A.R., Umbayev B., Sergazy S., Krivykh E., Gulyayev A., Nurgozhin T. 2020. Polyphenols as caloric-restriction mimetics and autophagy inducers in aging research. Nutrients. 12 (5), 1344. https://doi.org/10.3390/nu12051344
García-Aguilar A., Palomino O., Benito M., Guillén C. 2021. Dietary polyphenols in metabolic and neurodegenerative diseases: Molecular targets in autophagy and biological effects. Antioxidants (Basel). 10 (2), 142.https://doi.org/10.3390/antiox10020142
Maduro A.T., Luís C., Soares R. 2021. Ageing, cellular senescence and the impact of diet: An overview. Porto. Biomed. J. 6 (1), e120. https://doi.org/10.1097/j.pbj.0000000000000120
Pietrocola F., Lachkar S., Enot D.P., Niso-Santano M., Bravo-San Pedro J.M., Sica V., Izzo V., Maiuri M.C., Madeo F., Mariño G., Kroemer G. 2015. Spermidine induces autophagy by inhibiting the acetyltransferase EP300. Cell Death Differ. 2, 509–516. https://doi.org/10.1038/cdd.2014.215
Eisenberg T., Abdellatif M., Schroeder S., Primessnig U., Stekovic S., Pendl T., Harger A., Schipke J., Zimmermann A., Schmidt A., Tong M., Ruckenstuhl C., Dammbrueck C., Gross A.S., Herbst V., et al. 2016. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 22, 1428–1438. https://doi.org/10.1038/nm.4222
Finley J. 2018. Cellular stress and AMPK activation as a common mechanism of action linking the effects of metformin and diverse compounds that alleviate accelerated aging defects in Hutchinson–Gilford progeria syndrome. Med. Hypotheses. 118, 151–162. https://doi.org/10.1016/j.mehy.2018.06.029
Mariño G., Pietrocola F., Madeo F., Kroemer G. 2014. Caloric restriction mimetics: Natural/physiological pharmacological autophagy inducers. Autophagy. 10, 1879–1882. https://doi.org/10.4161/auto.36413
Escobar K.A., Cole N.H., Mermier C.M., VanDusseldorp A.T. 2019. Autophagy and aging: Maintaining the proteome through exercise and caloric restriction. Aging Cell. 18, e12876. https://doi.org/10.1111/acel.12876
Martin-Rincon M., Morales-Alamo D., Calbet J.A.L. 2018. Exercise-mediated modulation of autophagy in skeletal muscle. Scand. J. Med. Sci. Sports. 28, 772–781. https://doi.org/10.1111/sms.12945
Park S.S., Seo Y.K., Kwon K.-S. (2019. Sarcopenia targeting with autophagy mechanism by exercise. BMB Rep. 52, 64–69. https://doi.org/10.5483/BMBRep.2019.52.1.292
Babygirija R., Lamming D.W. (2021. The regulation of healthspan and lifespan by dietary amino acids. Transl. Med. Aging. 5, 17–30. https://doi.org/10.1016/j.tma.2021.05.001
Kim J.S., Choi H.W., Choi S., Do J.T. 2011. Reprogrammed pluripotent stem cells from somatic cells. Int. J. Stem Cells. 4 (1), 1–8. https://doi.org/10.15283/ijsc.2011.4.1.1
Jung H.-J, Tu Y., Yang S.H., Tatar A., Nobumori C., Wu D., Young S.G., Fong L.G. 2014. New LMNA knock-in mice provide a molecular mechanism for the ‘segmental aging’ in Hutchinson–Gilford progeria syndrome. Hum. Mol. Genet. 23, 1506–1515.
Nissan X., Blondel S., Navarro C., Maury Y., Denis C., Girard M., Martinat C., De Sandre-Giovannoli A., Levy N., Peschanski M. 2012. Unique preservation of neural cells in Hutchinson–Gilford progeria syndrome is due to the expression of the neural-specific miR-9 microRNA. Cell Rep. 2, 1–9. https://doi.org/10.1016/j.celrep.2012.05.015
Baek J.H., Schmidt E., Viceconte N., Strandgren C., Pernold K., Richard T.J., Van Leeuwen F.W., Dantuma N.P., Damberg P., Hultenby K., Ulfhake B., Mugnaini E., Rozell B., Eriksson M. 2015. Expression of progerin in aging mouse brains reveals structural nuclear abnormalities without detectible significant alterations in gene expression, hippocampal stem cells or behavior. Hum. Mol. Genet. 24, 1305–1321. https://doi.org/10.1093/hmg/ddu541
Jung H.-J., Coffinier C., Choe Y., Beigneux A.P., Davies B.S., Yang S.H., Barnes R.H. 2nd, Hong J., Sun T., Pleasure S.J., Young S.G., Fong L.G. 2012. Regulation of prelamin A but not lamin C by miR-9, a brain-specific microRNA. Proc. Natl. Acad. Sci. U. S. A. 109, E423–E431. https://doi.org/10.1073/pnas.1111780109
Schlachetzki J.C.M., Toda T., Mertens J. 2020. When function follows form: Nuclear compartment structure and the epigenetic landscape of the aging neuron. Exp. Gerontol. 133, 110876. https://doi.org/10.1016/j.exger.2020.110876
Yang S.H., Procaccia S., Jung H.J., Nobumori C., Tatar A., Tu Y., Bayguinov Y.R., Hwang S.J., Tran D., Ward S.M., Fong L.G., Young S.G. 2015. Mice that express farnesylated versions of prelamin a in neurons develop achalasia. Hum. Mol. Genet. 24, 2826–2840. https://doi.org/10.1093/hmg/ddv043
Dong X., Milholland B., Vijg J. 2016. Evidence for a limit to human lifespan. Nature. 538, 257–259. https://doi.org/10.1038/nature19793
Steenstrup T., Kark J.D., Verhulst S., Thinggaard M., Hjelmborg J.V.B., Dalgård C., Kyvik K.O., Christiansen L., Mangino M., Spector T.D., Petersen I., Kimura M., Benetos A., Labat C., Sinnreich R., et al. 2017. Telomeres and the natural lifespan limit in humans. Aging (Albany, NY). 9, 1130–1142.https://doi.org/10.18632/aging.101216
Tricola G.M., Simons M.J.P., Atema E., Boughton R.K., Brown J.L., Dearborn D.C., Divoky G., Eimes J.A., Huntington C.E., Kitaysky A.S., Juola F.A., Lank D.B., Litwa H.P., Mulder E.G.A., Nisbet I.C.T., et al. 2018. The rate of telomere loss is related to maximum lifespan in birds. Philos. Trans. R. Soc. Lond. B. 373 (1741), 20160445. https://doi.org/10.1098/rstb.2016.0445
Cawthon R.M., Smith K.R., O’Brien E., Sivatchenko A., Kerber R.A. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 361, 393–395. https://doi.org/10.1016/S0140-6736(03)12384-7
Aubert G., Lansdorp P.M. 2008. Telomeres and aging. Physiol. Rev. 88, 557–579. https://doi.org/10.1152/physrev.00026.2007
Celtikci. B., Erkmen G.K., Dikmen Z.G. 2020. Regulation and effect of telomerase and telomeric length in stem cells. Curr. Stem Cell Res. Ther. 16, 809–823. https://doi.org/10.2174/1574888X15666200422104423
Ros M., Carrascosa J.M. 2020. Current nutritional and pharmacological anti-aging interventions. Biochim. Biophys. Acta – Mol. Basis Dis. 1866 (3), 165612. https://doi.org/10.1016/j.bbadis.2019.165612
Vaiserman A, Krasnienkov D. 2021. Telomere length as a marker of biological age: State-of-the-art. Front. Genet. Open Issues. Future Perspectives. 21, 630186. https://doi.org/10.3389/fgene.2020.630186
Muñoz-Lorente M.A., Cano-Martin A.C., Blasco M.A. 2019. Mice with hyper-long telomeres show less metabolic aging and longer lifespans. Nat. Commun. 10 (1), 4723. https://doi.org/10.1038/s41467-019-12664-x
Froy H., Underwood S.L., Dorrens J., Seeker L.A., Watt K., Wilbourn R.V., Pilkington J.G., Harrington L., Pemberton J.M., Nussey D.H. 2021. Heritable variation in telomere length predicts mortality in Soay sheep. Proc. Natl. Acad. Sci. U. S. A. 118, e2020563118. https://doi.org/10.1073/pnas.2020563118
Wilkinson J.E., Burmeister L., Brooks S.V., Chan C.C., Friedline S., Harrison D.E., Hejtmancik J.F., Nadon N., Strong R., Wood L.K., Woodward M.A., Miller R.A. 2012. Rapamycin slows aging in mice. Aging Cell. 11, 675–682. https://doi.org/10.1111/j.1474-9726.2012.00832.x
Li Y.R., Li S., Lin C.C. 2018. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 44, 69–82. https://doi.org/10.1002/biof.1400
Weichhart T. 2018. mTOR as regulator of lifespan, aging, and cellular senescence: A mini-review. Gerontology. 64, 127–134. https://doi.org/10.1159/000484629
Blagosklonny M.V. 2019. Rapamycin for longevity: Opinion article. Aging (Albany, NY). 11, 8048–8067. https://doi.org/10.18632/aging.102355
Glossmann H.H., Lutz O.M.D. 2019. Metformin and aging. Gerontology. 65, 581–590. https://doi.org/10.1159/000502257
Bjedov I., Rallis C. 2020. The target of rapamycin signalling pathway in ageing and lifespan regulation. Genes (Basel). 11, 1043. https://doi.org/10.3390/genes11091043
Bernardes de Jesus B., Vera E., Schneeberger K., Tejera A.M., Ayuso E, Bosch F., Blasco M.A. 2012. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 4, 691–704. https://doi.org/10.1002/emmm.201200245
Boccardi V., Herbig U. 2012. Telomerase gene therapy: A novel approach to combat aging. EMBO Mol. Med. 4, 685–687. https://doi.org/10.1002/emmm.201200246
Bernardes de Jesus B., Schneeberger K., Vera E., Tejera A., Harley C.B., Blasco M.A. 2011. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 10, 604–621. https://doi.org/10.1111/j.1474-9726.2011.00700.x
Salvador L., Singaravelu G., Harley C.B., Flom P., Suram A., Raffaele J.M. 2016. A natural product telomerase activator lengthens telomeres in humans: A randomized, double blind, and placebo controlled study. Rejuvenation Res. 19, 478–484. https://doi.org/10.1089/rej.2015.1793
Tsoukalas D., Fragkiadaki P., Docea A.O., Alegakis A.K., Sarandi E., Thanasoula M., Spandidos D.A., Tsatsakis A., Razgonova M.P., Calina D. 2019. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol. Med. Rep. 20, 3701–3708. https://doi.org/10.3892/mmr.2019.10614
Prieto-Oliveira P. 2021. Telomerase activation in the treatment of aging or degenerative diseases: A systematic review. Mol. Cell. Biochem. 476 (2), 599–607. https://doi.org/10.1007/s11010-020-03929-x
Whittemore K., Vera E., Martínez-Nevado E., Sanpera C., Blasco MA. 2019. Telomere shortening rate predicts species life span. Proc. Natl. Acad. Sci. U. S. A., 116, 15122–15127. https://doi.org/10.1073/pnas.1902452116
Fernandez M.L., Thomas M.S., Lemos B.S., DiMarco D.M., Missimer A., Melough M., Chun O.K., Murillo A.G., Alyousef H.M., Medina-Vera I. 2018. TA-65, a telomerase activator improves cardiovascular markers in patients with metabolic syndrome. Curr. Pharm. Des. 24, 1905–1911. https://doi.org/10.2174/1381612824666180316114832
Ait-Ghezala G., Hassan S., Tweed M., Paris D., Crynen G., Zakirova Z., Crynen S., Crawford F. 2016. Identification of telomerase-activating blends from naturally occurring compounds. Altern. Ther. Health Med. 22, 6–14. PMID: .27433836
Berezutskii M.A., Durnova N.A., Vklasova Ya.A. 2019. Experimental and clinical studies on the mechanisms of anti-aging effects of chemical compounds from Astragalus membranaceus: A review. Usp Gerontol. 32, 702–710.
Sharma R., Martins N. 2020. Telomeres, DNA damage and ageing: Potential leads from ayurvedic rasayana (anti-ageing). drugs. J. Clin. Med. 9 (8), 2544. https://doi.org/10.3390/jcm9082544
Alshinnawy A.S., El-Sayed W.M., Taha A.M., Sayed A.A., Salem A.M. 2020. Astragalus membranaceus and Punica granatum alleviate infertility and kidney dysfunction induced by aging in male rats. Turk. J. Biol. 44, 166–175. https://doi.org/10.3906/biy-2001-5
Bernardes de Jesus B., Blasco M.A. 2013. Telomerase at the intersection of cancer and aging. Trends Genet. 29, 513–520. https://doi.org/10.1016/j.tig.2013.06.007
Yang F., Xiu M., Yang S., Li X., Tuo W., Su Y., He J., Liu Y. 2021. extension of drosophila lifespan by astragalus polysaccharide through a mechanism dependent on antioxidant and insulin/IGF-1 signaling. Evid. Based Complement. Alternat. Med. 2021, 6686748. https://doi.org/10.1155/2021/6686748.9999
Shan H., Zheng X., Li M. 2019. The effects of astragalus membranaceus active extracts on autophagy-related diseases. Int. J. Mol. Sci. 20 (8), 1904. https://doi.org/10.3390/ijms20081904
Zhang X., Liang T., Yang W., Zhang L., Wu S., Yan C., Li Q. 2020. Astragalus membranaceus injection suppresses production of interleukin-6 by activating autophagy through the AMPK-mTOR pathway in lipopolysaccharide-stimulated macrophages. Oxid. Med. Cell. Longev. 2020, 1364147. https://doi.org/10.1155/2020/1364147
Harley C.B., Liu W., Flom PL., Raffaele J.M. 2013. A natural product telomerase activator as part of a health maintenance program: Metabolic and cardiovascular response. Rejuvenation Res. 16, 386–395. https://doi.org/10.1089/rej.2013.1430
Liu P., Zhao H., Luo Y. 2017. Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known chinese tonic. Aging Dis. 8, 868–886.https://doi.org/10.14336/AD.2017.0816
Maier R., Bawamia B., Bennaceur K., Dunn S., Marsay L., Amoah R., Kasim A., Filby A., Austin D., Hancock H., Spyridopoulos I. 2020. Telomerase activation to reverse immunosenescence in elderly patients with acute coronary syndrome: Protocol for a randomized pilot trial. JMIR Res. Protoc. 9, e19456. https://doi.org/10.2196/19456
Yegorov Y.E. 2020. Healthy aging: Antioxidants, uncouplers and/or telomerase? Mol. Biol. (Moscow). 54 (3), 311–316.
Pignatti C., D’Adamo S., Stefanelli C., Flaigni F., Cetrullo S. 2020. Nutrients and pathways that regulate health span and life span. Geriatrics (Basel). 5 (4), 95. https://doi.org/10.3390/geriatrics5040095
Ukraintseva S., Arbeev K., Duan M., Akushevich I., Kulminski A., Stallard E., Yashin A. 2021. Decline in biological resilience as key manifestation of aging: Potential mechanisms and role in health and longevity. Mech. Ageing Dev. 194, 111418. https://doi.org/10.1016/j.mad.2020.111418
Yu M., Zhang H., Wang B., Zhang Y., Zheng X., Shao B., Zhuge Q., Jin K. 2021. Key signaling pathways in aging and potential interventions for healthy aging. Cells. 10 (3), 660. https://doi.org/10.3390/cells10030660
Gorbunova V., Seluanov A. 2009. Coevolution of telomerase activity and body mass in mammals: From mice to beavers. Mech. Ageing Dev. 130 (1–2), 3–9. https://doi.org/10.1016/j.mad.2008.02.008
Abegglen L.M., Caulin A.F., Chan A., Lee K., Robinson R., Campbell M.S., Kiso W.K., Schmitt D.L., Waddell P.J., Bhaskara S., Jensen S.T., Maley C.C., Schiffman J.D. 2015. Potential mechanisms for cancer resistance in elephants and comparative cellular response to DNA damage in humans. J. Am. Med. Assoc. 314, 1850–1860. https://doi.org/10.1001/jama.2015.13134
Ruby J.G., Smith M., Rochelle Buffenstein R. 2018. Naked mole-rat mortality rates defy gompertzian laws by not increasing with age. eLife. 7, e31157. https://doi.org/10.7554/eLife.31157
Seluanov A., Gladyshev V.N., Vijg J., Gorbunova V. 2018. Mechanisms of cancer resistance in long-lived mammals. Nat. Rev. Cancer. 18 (7), 433–441. https://doi.org/10.1038/s41568-018-0004-9
Takasugi M., Firsanov D., Tombline G., Ning H., Ablaeva J., Seluanov A., Gorbunova V. 2020. Naked mole-rat very-high-molecular-mass hyaluronan exhibits superior cytoprotective properties. Nat. Commun. 11, 2376. https://doi.org/10.1038/s41467-020-16050-w
Zhao S., Lin L., Kan G., Xu C., Tang Q., Yu C., Cui S. 2014). High autophagy in the naked mole rat may play a significant role in maintaining good health. Cell. Physiol. Biochem. 33 (2), 321–332. https://doi.org/10.1159/000356672
Brassard J.A. Fekete N., Garnier A., Hoesli C.A. 2016. Hutchinson–Gilford progeria syndrome as a model for vascular aging. Biogerontology. 17, 129–145. https://doi.org/10.1007/s10522-015-9602-z
Smith E.S.J., Park T.J., Holmes M.M., Buffenstein R. 2021. Some exciting future directions for work on naked mole-rats. Adv. Exp. Med. Biol. 1319, 409–420. https://doi.org/10.1007/978-3-030-65943-1_17
Macicior J., Marcos-Ramiro B., Ortega-Gutiérrez S. 2021. Small-molecule therapeutic perspectives for the treatment of progeria. Int. J. Mol Sci. 22 (13), 7190. https://doi.org/10.3390/ijms22137190
Cabral W.A., Tavarez U.L., Beeram I., Yeritsyan D., Boku Y.D., Eckhaus M.A., Nazarian A., Erdos M.R., Collins F.S. 2021. Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson–Gilford progeria syndrome. Aging Cell. 20 (9), e13457. https://doi.org/10.1111/acel.13457
Kychygina A., Dall’Osto M., Allen J.A.M., Cadoret J.C., Piras V., Pickett H.A., Crabbe L. 2021. Progerin impairs 3D genome organization and induces fragile telomeres by limiting the dNTP pools. Sci. Rep. 11 (1), 13195. https://doi.org/10.1038/s41598-021-92631-z
Coppedè F. 2021. Mutations involved in premature-ageing syndromes. Appl. Clin. Genet. 14, 279–295. https://doi.org/10.2147/TACG.S273525
Yu M., Zhang H., Wang B., Zhang Y., Zheng X., Shao B., Zhuge Q., Jin K. 2021. Key signaling pathways in aging and potential interventions for healthy aging. Cells. 10 (3), 660. https://doi.org/10.3390/cells10030660
Cabral W.A., Tavarez U.L., Beeram I., Yeritsyan D., Boku Y.D., Eckhaus M.A., Nazarian A., Erdos M.R., Collins F.S. 2021. Genetic reduction of mTOR extends lifespan in a mouse model of Hutchinson–Gilford progeria syndrome. Aging Cell. 20 (9), e13457. https://doi.org/10.1111/acel.13457
Chen N.Y., Kim P.H., Fong L.G., Young S.G. 2020. Nuclear membrane ruptures, cell death, and tissue damage in the setting of nuclear lamin deficiencies. Progress and trends. Nucleus. 11, 237–249. https://doi.org/10.1080/19491034.2020.1815410
Dreesen O. 2020. Towards delineating the chain of events that cause premature senescence in the accelerated aging syndrome Hutchinson–Gilford progeria (HGPS). Biochem. Soc. Trans. 48, 981–991. https://doi.org/10.1042/BST20190882
ACKNOWLEDGMENTS
I am grateful to S.N. Pchelina for attention to the review and helpful comments, O.M. Gorbenko for help in manuscript preparation and illustrations, and F.V. Gorbenko for valuable information.
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 14-04-00587-a, to M.I. Mosevitsky).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The author declares that he has no conflict of interest.
This work does not contain any studies involving animals or human subjects performed by the author.
Additional information
Translated by T. Tkacheva
Rights and permissions
About this article
Cite this article
Mosevitsky, M.I. Progerin and Its Role in Accelerated and Natural Aging. Mol Biol 56, 125–146 (2022). https://doi.org/10.1134/S0026893322020091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893322020091