Abstract
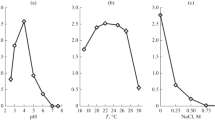
An acidophilic heterotrophic gram-negative Acidiphilium symbioticum strain H8 is an important bacterium possessing a high capacity for heavy metal binding. As these bacteria inhabit acidic mine regions they are subjected to occasional temperature stress; thus their membrane profile is very important. Cell morphology determined by scanning and transmission electron microscopy revealed the surface and internal adaptation of the organelles. Phosphatidyl ethanolamine, phosphatidyl inositol, and phosphatidic acid are the major phospholipids affected by growth temperature. The membrane fluidity in response to temperature stress was investigated by measuring fluorescence anisotropy using 1,6-diphenyl-1,3,5-hexatriene (DPH) as the probe. Significant changes in membrane fluidity revealed the transition temperature midpoints as 11 and 38°C. To maintain the optimum fluidity increasing the percentage of cis-vaccenic acid at suboptimal and palmitic acid at the upper cardinal temperature support the evidence of membrane remodeling in different growth temperatures. The protein profile from SDS-PAGE provided information about different polypeptide bands which may be responsible for the different metal binding abilities of the strain.




Similar content being viewed by others
REFERENCES
Aldsworth, T.G., Sharman, R.L., and Dodd, C.E., Bacterial suicide through stress, Cell, Mol. Life Sci., 1999, vol. 56, pp. 378‒383.
Bakholdina, S., Sanina, N., and Krasikova, I., The impact of abiotic factors (temperature and glucose) on physicochemical properties of lipids from Yersinia pseudotuberculosi, Biochimie, 2004, vol. 86, pp. 875‒881.
Barker, H.A. and Kornberg, A., The structure of the adenosine triphosphate of Thiobacillus thiooxidans, J. Bacteri-ol., 1954, vol. 68, pp. 655‒661.
Beveridge, T.J. and Graham, L.L., Surface layers of bacteria, Microbiol. Rev., 1991, vol. 55, pp. 684‒705.
Bhattacharya, S., Chakrabarti, B.K., Das, A., Kundu, P.N., and Banerjee, P.C., Acidiphilium symbioticum sp. nov., an acidophilic heterotrophic bacterium from Thiobacillus ferrooxidans cultures isolated from Indian mines, Can. J. Microbiol., 1991, vol. 37, pp. 78‒85.
Bligh, E.G. and Dyer, W.J., A rapid method for total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, vol. 37, pp. 911‒917.
Bohuszewicz, O., Liu, J., and Low, H.H., Membrane remodeling in bacteria, J. Struct. Biol., 2016, vol. 196, pp. 3‒14.
Brown, J.L., Ross, T., McMeekin, T.A., and Nichols, P.D., Acid habituation of Escherichia coli and the potential role of cyclopropane fatty acids in low pH tolerance, Int. J. Food Microbiol., 1997, vol. 37, pp. 163‒173.
Brown, N.L., Barrett, S.R., Camakaris, J., Lee, B.T., and Rouch, D.A., Molecular genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli plasmid pRJ1004, Mol. Microbiol., 1995, vol. 17, pp. 1153‒1166.
Chakravarty, R. and Banerjee, P.C., Mechanism of cadmium binding on the cell wall of an acidophilic bacterium, Bioresour. Technol., 2012, vol. 108, pp. 176‒183.
Chakravarty, R. and Banerjee, P.C., Morphological changes in an acidophilic bacterium induced by heavy metals, Extremophiles, 2008, vol. 12, pp. 279‒284.
Davydova, L., Bakholdina, S., Barkina, M., Velansky, P., Bogdanov, M., and Sanina, N., Effects of elevated growth temperature and heat shock on the lipid composition of the inner and outer membranes of Yersinia pseudotuberculosis, Biochimie, 2016, vol. 123, pp. 103‒109.
Denich, T.J., Beaudette, L.A., Lee, H., and Trevors, J.T., Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes, J. Microbiol. Methods, 2003, vol. 52, pp. 149‒182.
Dombek, K.M. and Ingram, L.O., Effects of ethanol on the Escherichia coli plasma membrane, J. Bacteriol., 1984, vol. 157, pp. 233‒239.
Dufourc, E.J., Smith, I.C.P., and Jarrell, H.C., The role of cyclopropane moieties in the lipid properties of biological membranes: a deuterium NMR structural and dynamical approach, Biochemistry, 1984, vol. 23, pp. 2300‒2309.
Eisenberg, A.D. and Corner, T.R., Osmotic behavior of bacterial protoplasts: Temperature effects, J. Bacteriol., 1973, vol. 114, pp. 1177‒1183.
Guiliani, N. and Jerez, C.A., Molecular cloning, sequencing, and expression of omp-40, the gene coding for the major outer membrane protein from the acidophilic bacterium Thiobacillus ferrooxidans, Appl. Environ. Microbiol., 2000, vol. 66, pp. 2318‒2324.
Hasegawa, Y., Kawada, N., and Nosoh, Y., Change in chemical composition of membrane of Bacillus caldotenax after shifting the growth temperature, Arch. Microbiol., 1980, vol. 126, pp. 103‒108.
Heyde, M. and Portalier, R., Regulation of major outer membrane porin proteins of Escherichia coli K 12 by pH, Mol. Gen. Genet., 1987, vol. 208, pp. 511‒517.
Itoh, S., Iwaki, M., Wakao, N., Yoshizu, K., Aoki, A., and Tazaki, K., Accumulation of Fe, Cr and Ni metals inside cells of acidophilic bacterium Acidiphilium rubrum that produces Zn-containing bacteriochlorophyll a, Plant Cell Physiol., 1998, vol. 39, pp. 740‒744.
Jorge, C.D., Borges, N., and Santos, H., A novel pathway for the synthesis of inositol phospholipids uses CDP-inositol as donor of the polar head group, Environ. Microbiol., 2015, vol. 17, pp. 2492—2504.
Kannan, N., Rao, A.S., and Nair, A., Microbial production of omega-3 fatty acids: an overview, J. Appl. Microbiol., 2021, vol. 131, pp. 2114—2130.
Kerger, B.D., Nichols, P.D., Antworth, C.P., Sand, W., Bock, E., Cox, J.C., Langworthy, T.A., and White, D.C., Signature fatty acids in the polar lipids of acid-producing Thiobacillus spp.: methoxy, cyclopropyl, alpha-hydroxy-cyclopropyl and branched and normal monoenoic fatty acids, FEMS Microbiol. Lett., 1986, vol. 38, pp. 67‒77.
Kishimoto, N., Kosako, Y., and Tano, T., Acidiphilium aminolytica sp. nov.: an acidophilc chemoorganotrophic bacterium isolated from acidic mineral environment, Curr. Microbiol., 1993, vol. 27, pp. 131‒136.
Liesegang, H., Lemke, K., Siddiqui, R.A., and Schlege, H.G., Characterization of the inducible nickel and cobalt resistance determinant cnr from pMOL28 of Alcaligene seutrophus CH34, J. Bacteriol., 1993, vol. 175, pp. 767‒778.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., and Randall, R.J., Protein measurement with the Folin phenol reagent, J. Biol. Chem., 1951, vol. 193, pp. 265‒275.
Lundgren, D.G., Anderson, K.J., Remsen, C.C., and Mahoney, R.P., Culture, structure and physiology of the chemoautotrophic bacterium Ferrobacillus ferrooxidans, Develop. Ind. Microbiol., 1964, vol. 6, pp. 250‒257.
Mahapatra, N.R. and Banerjee, P.C., Extreme tolerance to cadmium and high resistance to copper, nickel and zinc in different Acidiphilium strains, Lett. Appl. Microbiol., 1996, vol. 23, pp. 393‒397.
Matlakowska, R., Skudlarska. E., and Skłodowska, A., The growth, ferrous iron oxidation and ultrastructure of Acidithiobacillus ferrooxidans in the presence of dibutyl phthalate, Pol. J. Microbiol., 2006, vol. 55, pp. 203‒210.
Matsuzawa, Y., Kanbe, T., Suzuki, J., and Hiraishi, A., Ultrastructure of the acidophilic aerobic photosynthetic bacterium Acidiphilium rubrum, Curr. Microbiol., 2000, vol. 40, pp. 398‒401.
Murínová, S. and Dercová, K., Response mechanisms of bacterial degraders to environmental contaminants on the level of cell walls and cytoplasmic membrane, Int. J. Microbiol., 2014, vol. 2014, pp. 1‒16.
Mykytczuk, N.C.S., Trevors, J.T., Leduc, L.G., and Ferroni, G.D., Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress, Prog. Biophys. Mol. Biol., 2007, vol. 95, pp. 60‒82.
Neumann, G., Veeranagouda, Y., Karegoudar, T.B., Sahin, Ö., Mäusezahl, I., Kabelitz, N., Kappelmeyer, U., and Heipieper, H.J., Cells of Pseudomonas putida and Enterobacter sp. adapt to toxic organic compounds by increasing their size, Extremophiles, 2005, vol. 9, pp. 163‒168.
Nies, D.H., Efflux mediated heavy metal resistance in prokaryotes, FEMS Microbiol. Rev., 2003, vol. 27, pp. 313‒339.
Pal, S., Banik, S.P., Ghorai, S., Chowdhury, S., and Khowala, S., Purification and characterization of a thermostable intra-cellular beta-glucosidase with trans glycosylation properties from filamentous fungus Termitomyces clypeatus, Bioresour. Technol., 2010, vol. 101, pp. 2412‒2420.
Peng, H., Yi, L., Zhang, X., Xiao, Y., Gao, Y., and He, C., Changes in the membrane fatty acid composition in Anoxybacillus flavithermus sub sp. yunnanensis E13 T as response to solvent stress, Arch. Microbiol., 2017, vol. 199, pp. 1‒8.
Pidcock, E. and Moore, G.R., Structural characteristics of protein binding sites for calcium and lanthanide ions, J. Bi-ol. Inorg. Chem., 2001, vol. 6, pp. 479‒489.
Rodríguez, M., Campos, S., and Gómez-Silva, B., Studies on native strains of Thiobacillus ferrooxidans. III. Studies on the surface of Thiobacillus ferrooxidans. Characterization of the lipopolysaccharide and some proteins, Biotechnol. Appl. Biochem., 1986, vol. 8, pp. 292‒299.
Shinitzky, M. and Barenholz, Y., Fluidity parameters of lipid regions determined by fluorescence polarization, Biochim. Biophys. Acta, 1978, vol. 515, pp. 367‒394.
Singh, S.K., Singh, A., and Banerjee, P.C., Plasmid encoded AcrAB–TolC tripartite multidrug-efflux system in Acidiphilium symbioticum H8, Curr. Microbiol., 2010, vol. 61, pp. 163‒168.
Sohlenkamp, C. and Geiger, O., Bacterial membrane lipids: diversity in structures and pathways, FEMS Microbiol. Rev., 2016, vol. 40, pp. 133‒159.
Sohlenkamp, C., L’opez-Lara, I.M., and Geiger, O., Biosynthesis of phosphatidylcholine in bacteria, Prog. Lipid. Res., 2003, vol. 42, pp. 115‒162.
Souza, K.A., Kostiw, L.L., and Tyson, B.J., Alteration in normal fatty acid composition in a temperature sensitive mutant of a thermophilic Bacillus, Arch. Microbiol., 1974, vol. 97, pp. 89‒102.
Spratt, B.G., Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12, Proc. Natl. Acad. Sci. U. S. A., 1975, vol. 72, pp. 2999‒3003.
Suutari, M. and Laakso, S., Microbial fatty acids and thermal adaptation, Crit. Rev. Microbiol., 1994, vol. 20, pp. 285‒328.
Talaro, K.P. and Talaro, A., Foundations in Microbiology, McGraw Hill, 1999, 3rd ed.
Wada, M., Fukunaga, N., and Sasaki, S., Aerobic synthesis of unsaturated fatty acids in a psychrotrophic bacterium, Pseudomonas sp. strain E-3, having two mechanisms for unsaturated fatty acid synthesis, J. Gen. Appl. Microbiol., 1991, vol. 37, pp. 355 ‒362.
Wakao, N., Nagasawa, N., Matsuura, T., Matsukura, H., Matsumoto, T., Hiraishi, A., Sakurai, Y., and Shiota, H., Acidiphilium multivorum sp. nov., an acidophilic, chemoorganotrophic bacterium from pyritic acid mine drainage, J. Gen. Appl. Microbiol., 1994, vol. 40, pp. 143‒159.
Wichlacz, P.L., Unz, R.F., and Langworthy, T.A., Acidiphilium angustum sp. nov., Acidiphilium facilis sp. nov., and Acidiphilium rubrum sp. nov.: acidophilic heterotrophic bacteria isolated from acidic coal mine drainage, Int. J. Syst. Bacteriol., 1986, vol. 36, pp. 197‒201.
Wisdom, C. and Welker, N.E., Membranes of Bacillus stearothermophilus: factors affecting protoplast stability and thermostability of alkaline phosphatase and reduced nicotin amide adenine dinucleotide oxidase, J. Bacteriol., 1973, vol. 114, pp. 1336−1345.
Yao, J., and Rock, C.O., Phosphatidic acid synthesis in bacteria, Biochim. Biophys. Acta, 2013, vol. 1831, pp. 495—502.
Yasuda, M., Oyaizu, H., Yamagishi, A., and Oshima, T., Morphological variation of new Thermoplasma acidophilum isolates from Japanese hot springs, Appl. Environ. Mic-robiol., 1995, vol. 61, pp. 3482‒3485.
Young, K.D., The selective value of bacterial shape, Microbiol. Mol. Biol. Rev., 2006, vol. 70, pp 660‒703.
ACKNOWLEDGMENTS
We gratefully acknowledge the help of Dr. S. Cha-kraborty, University Science Instrumentation Centre, Burdwan University, West Bengal, for providing SEM facilities and Dr. T.C. Nag, All India Institute of Medical Science, New Delhi for providing TEM facilities. Many thanks are also addressed to Dr. A. Sen, Indian Institute of Chemical Biology, Kolkata, for his support in providing a GC-MS facility. R. Chakravarty acknowledges the Research Fellowship provided by the Council of Scientific and Industrial Research (CSIR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies involving animals or human participants performed by any of the authors.The authors declare that they have no conflicts of interest.
Supplementary Information
Rights and permissions
About this article
Cite this article
Chakravarty, R., Banerjee, P.C. Survival of a Novel Bacterium Acidiphilium symbioticum H8 under Thermal Stress. Microbiology 92, 534–544 (2023). https://doi.org/10.1134/S0026261722602457
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722602457