Abstract
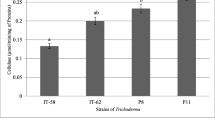
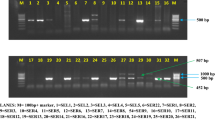
Endolichenic fungi are considered a promising source of new materials. For further evaluation of some biological activities of the Trichoderma strains isolated from lichens Dirinaria spp. and Cryptothecia spp., their antifungal and antibacterial activities were screened by the methods of dual culture and environmental toxicity. Substrate degradation was evaluated using the qualitative enzyme assays. Fourteen strains of Trichoderma spp. were isolated from 60 lichen samples. All the isolates were able to inhibit the radial growth of tested fungal strains (Bipolaris spp., Colletotrichum spp., Corynespora cassiicola, and Fusarium spp.). Otherwise, only 12/14 isolates were found capable of competing for substrates with Ralstonia solanacearum. The cell-free supernatant obtained from the cultures possessed both antifungal and antibacterial activities. The antagonistic activity of the isolates was selective. Most of the isolates were able to degrade at least one of the investigated substrates, namely cellulose, pectin, and starch. All strains could produce peroxidase; none of the isolates possessed laccase and tyrosinase. A potential antagonistic fungal strain VDT6 has been identified as Trichoderma harzianum. The assessment results indicated that the Trichoderma isolates could be used in agriculture as biological control agents.




Similar content being viewed by others
REFERENCES
Al-Samarrai, T.H. and Schmid, J., A simple method for extraction of fungal genomic DNA, Lett. Appl. Microbiol., 2000, vol. 30, pp. 53–56. https://doi.org/10.1046/j.1472-765x.2000.00664.x
Chaverri, P. and Samuels, G.J., Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): Species with Green Ascospores, Utrecht: Centralbureau voor Schimmelcultures, 2003.
Eggins, H.O.W. and Pugh, G.J.F., Isolation of cellulose-decomposing fungi from the soil, Nature, 1962, vol. 193, pp. 94–95. https://doi.org/10.1038/193094a0
Elix, J.A., Cryptothecia, in Flora of Australia. Volume 57: Lichens 5, McCarthy, P.M. and Kuchlmayr, B., Eds., Victoria: CSIRO/ABRS, 2009a, pp. 1–12.
Elix, J.A., Dirinaria, in Flora of Australia. Volume 57: Lichens 5, McCarthy, P.M. and Kuchlmayr, B., Eds., Victoria: CSIRO Publishing/ABRS, 2009b, pp. 509–517.
Ficke, A., Cowger, C., Bergstrom, G., and Brodal, G., Understanding yield loss and pathogen biology to improve disease management: Septoria nodorum blotch—a case study in wheat, Plant Dis., 2018, vol. 102, pp. 696–707. https://doi.org/10.1094/PDIS-09-17-1375-FE
Fujita, S.I., Senda, Y., Nakaguchi, S., and Hashimoto, T., Multiplex PCR using internal transcribed spacer 1 and 2 regions for rapid detection and identification of yeast strains, J. Clin. Microbiol., 2001, vol. 39, pp. 3617–3622. https://doi.org/10.1128/JCM.39.10.3617-3622.2001
Girlanda, M., Isocrono, D., Bianco, C., and Luppi-Mosca, A.M., Two foliose lichens as microfungal ecological niches, Mycologia, 2008, vol. 89, pp. 531–536. https://doi.org/10.1080/00275514.1997.12026814
Hamzah, T.N.T., Lee, S.Y., Hidayat, A., Terhem, R., Faridah-Hanum, I., and Mohamed, R., Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, Fusarium solani, Front. Microbiol., 2018, vol. 9, p. 1707. https://doi.org/10.3389/fmicb.2018.01707
Hong, N.V., Hien, N.T., Minh, N.T.T., and Toan, H.C., Forecasting saline intrusion under the influence of the northeast monsoon in the Mekong Delta, VN J. Hydrometeorol., 2021, vol. 9, pp. 23–36. https://doi.org/10.36335/VNJHM.2021(9).23-36
Kannangara, B.T., Rajapaksha, R.S., and Paranagama, P.A., Nature and bioactivities of endolichenic fungi in Pseudocyphellaria sp., Parmotrema sp. and Usnea sp. at Hakgala montane forest in Sri Lanka, Lett. Appl. Microbiol., 2009, vol. 48, pp. 203–209. https://doi.org/10.1111/j.1472-765X.2008.02512.x
Karthikaidevi, G., Thirumaran, G., Manivannan, K., Anantharaman, P., Kathiresan, K., and Balasubaramanian, T., Screening of the antibacterial properties of lichen Roccella belangeriana (Awasthi) from Pichavaram mangrove (Rhizophora sp.), Adv. Biol. Res., 2009, vol. 3, pp. 127–131.
Kellogg, J.J. and Raja, H.A., Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon, Phytochem. Rev., 2016, vol. 16, pp. 271–293. https://doi.org/10.1007/s11101-016-9473-1
Li, Y., Sun, R., Yu, J., Saravanakumar, K., and Chen, J., Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum, Indian J. Microbiol., 2016, vol. 56, pp. 318–327. https://doi.org/10.1007/s12088-016-0581-9
Mejía, L.C., Rojas, E.I., Maynard, Z., Bael, S.V., Arnold, A.E., Hebbar, P., Samuels, G.J., Robbins, N., and Herre, E.A., Endophytic fungi as biocontrol agents of Theobroma cacao pathogens, Biol. Control, 2008, vol. 46, pp. 4–14. https://doi.org/10.1016/j.biocontrol.2008.01.012
Nedialkova, D. and Naidenova, M., Screening the antimicrobial activity of actinomycetes strains isolated from Antarctica, J. Cult. Collect., 2005, vol. 4, pp. 29–35.
Oh, S.Y., Yang, J.H., Woo, J.J., Oh, S.O., and Hur, J.S., Diversity and distribution patterns of endolichenic fungi in Jeju Island, South Korea, Sustainability, 2020, vol. 12, p. 3769. https://doi.org/10.3390/su12093769
Padhi, S. and Tayung, K., In vitro antimicrobial potentials of endolichenic fungi isolated from thalli of Parmelia lichen against some human pathogens, Beni-Suef Univ. J. Basic Appl. Sci., 2015, vol. 4, pp. 299–306. https://doi.org/10.1016/j.bjbas.2015.11.006
Petrini, O., Hake, U., and Dreyfuss, M.M., An analysis of fungal communities isolated from fruticose lichens, Mycologia, 1990, vol. 82, pp. 444–451. https://doi.org/10.1080/00275514.1990.12025907
Stalpers, J.A., Identification of Wood-Inhabiting Fungi in Pure Culture, Baarn: Centraalbureau voor Schimmelcultures, 1978.
Suryanarayanan, T.S. and Thirunavukkarasu, N., Endolichenic fungi: the lesser known fungal associates of lichens, Mycology, 2017, vol. 8, pp. 189–196. https://doi.org/10.1080/21501203.2017.1352048
Tripathi, M. and Joshi, Y., Endolichenic Fungi: Present and Future Trends, Singapore: Springer, 2019.
Verma, M., Brar, S.K., Tyagi, R.D., Surampalli, R.Y., and Valéro, J.R., Antagonistic fungi, Trichoderma spp.: panoply of biological control, Biochem. Eng. J., 2007, vol. 37, pp. 1–20. https://doi.org/10.1016/j.bej.2007.05.012
Wheeler, K.A. and Hocking, A.D., Interactions among xerophilic fungi associated with dried salted fish, J. Appl. Bacteriol., 1993, vol. 74, pp. 164–169. https://doi.org/10.1111/j.1365-2672.1993.tb03010.x
Yang, J.H., Oh, S.Y., Kim, W., Woo, J.J., Kim, H., and Hur, J.S., Effect of isolation conditions on diversity of endolichenic fungal communities from a foliose lichen, Parmotrema tinctorum, J. Fungi (Basel), 2021, vol. 7, p. 335. https://doi.org/10.3390/jof7050335
Yuliar, Nion, Y.A., and Toyota, K., Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum, Microbes Environ., 2015, vol. 30, pp. 1–11. https://doi.org/10.1264/jsme2.ME14144
ACKNOWLEDGMENTS
N.T. Phu was funded by Vingroup Joint Stock Company and supported by the Domestic Master/Ph.D. Scholarship Programme of Vingroup Innovation Foundation, Institute of Big Data (VNCDLL), code VINIF.2021.ThS.61. The authors would like to acknowledge the Vingroup Innovation Foundation, Institute of Big Data for the financial support.
Funding
This study was supported by the Vingroup Joint Stock Company and the Domestic Master/Ph.D (Project/grant number VINIF.2021.ThS.61), Vietnam.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Phu, N.T., Cam, V.T., Minh, N.T. et al. Biological Activity of the Endolichenic Trichoderma spp. Isolated from Lichens Cryptothecia spp. and Dirinaria spp.. Microbiology 92, 408–417 (2023). https://doi.org/10.1134/S0026261722602093
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261722602093