Abstract
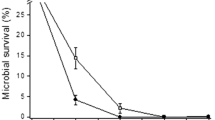
A comparative response of marine bacteria Pseudomonas pseudoalcaligenes NP103 and P. aeruginosa N6P6 under pH stress and UV radiation (UVR) revealed that both their survival pattern and repair mechanism are species specific. In case of P. pseudoalcaligenes NP103, the survival was maximum at pH 8, which decreased with decline in pH of the medium. Whereas, in P. aeruginosa N6P6, maximum survival was observed at pH 7. On exposure to UVR at different doses (25–200 mJ/cm2) and increasing concentrations of Na+ (1–6%), considerable differences in the recovery (2% for P. pseudoalcaligenes NP103 and 3% for P. aeruginosa N6P6) from UVR induced damage was observed. The qRT-PCR analysis of DNA repair genes (recA and uvrA) of marine bacteria subjected to different pH conditions showed significant (P < 0.05) up-regulation of both genes at pH 6, indicating higher degree of DNA damage at low pH. Furthermore, exposure of UVR irradiated cell suspensions to visible light exhibited greater photo-reactivating capacity in P. pseudoalcaligenes NP103 as compared to P. aeruginosa N6P6. The present findings indicate that pH and UVR exposure have crucial role in dictating the light dependent and independent DNA repair pathway in marine bacteria. Further, we speculate that both these repair response to the environmental stressors varies with bacterial species.
Similar content being viewed by others
References
Arrieta, J.M., Weinbauer, M.G., and Herndl, G.J., Interspecific variability in sensitivity to UVradiation and subsequent recovery in selected isolates of marine bacteria, Appl. Environ. Microbiol., 2000, vol. 66, no.4, pp. 1468–1473.
Booth, M.G., Jeffrey, W.H., and Miller R.V., RecA expression in response to solar UVR in the marine bacterium, Vibrio natriegens, Microb. Ecol., 2001, vol. 42, no. 4, pp. 531–539.
Chakraborty, J. and Das, S., Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11, Environ. Sci. Pollut. R., 2014, vol. 21, pp. 14188–14201.
Collins, A.R., The comet assay for DNA damage and repair, Mol. Biotechnol., 2004, vol. 26, no. 3, pp. 249–261.
Crowley, D.J. and Hanawalt, P.C., Induction of the SOS response increases the efficiency of global nucleotide excision repair of cyclobutane pyrimidine dimers, but not 6-4 photoproducts, in UV-irradiated Escherichia coli, J. Bacteriol., 1998, vol. 180, no. 13, pp. 3345–3352.
Dash, H.R., Mangwani, N., and Das, S., Characterization and potential application in mercury bioremediation of highly mercury-resistant marine bacterium Bacillus thuringiensis PW-05, Environ. Sci Pollut. R., 2014, vol. 21, no. 4, pp. 2642–2653.
Dash, H.R., Mangwani, N., Chakraborty, J., Kumari, S., and Das, S., Marine bacteria: potential candidates for enhanced bioremediation, Appl. Microbiol. Biotechnol., 2013, vol. 97, no. 2, pp. 561–571.
Eisen, J.A., and Hanawalt, P.C., A phylogenomic study of DNA repair genes, proteins, and processes, Mutat. Res., 1999, vol. 435, no. 3, pp. 171–213.
Imamura, M., Harada, K., Sawada, S., Akagi, K., and Ohnishi, T., Damage to DNA purified from the radioresistant prokaryote, Deinococcus radiodurans, by acid heating, Int. J. Mol. Med., 1999, vol. 3, pp. 391–395.
Jaciuk, M., Nowak, E., Skowronek, K., Tanska, A., and Nowotny, M., Structure of UvrA nucleotide excision repair protein in complex with modified DNA, Nat. Struct. Mol. Biol., 2011, vol. 18, pp. 191–197.
Janion, C., Inducible SOS response system of DNA repair and mutagenesis in Escherichia coli, Int. J. Biol. Sci., 2008, vol. 4, no. 6, pp. 338–344.
Joux, F., Jeffrey, W.H., Lebaron, P., and Mitchell, D.L., Marine bacterial isolates display diverse responses to UV-B radiation, Appl. Environ. Microbiol., 1999, vol. 65, no. 9, pp. 3820–3827.
Krause, S., Liebetrau, V., Gorb, S., Sánchez-Román, M., McKenzie, J.A., and Treude, T., Microbial nucleation of Mg-rich dolomite in exopolymeric substances under anoxic modern seawater salinity: New insight into an old enigma, Geology, 2012, vol. 40, no. 7, pp. 587–590.
Lee, R.F. and Steinert, S., Use of the single cell gel electrophoresis/comet assay for detecting DNA damage in aquatic (marine and freshwater) animals, Mutat. Res., 2003, vol. 544, no. 1, pp. 43–64.
Lenhart, J.S., Schroeder, J.W., Walsh, B.W., and Simmons, L.A., DNA repair and genome maintenance in Bacillus subtilis, Microbiol. Mol. Biol. Rev., 2012, vol. 76, no. 3, pp. 530–564.
Mangoli, S., Rath, D., Goswami, M., and Jawali, N., Increased ultraviolet radiation sensitivity of Escherichia coli grown at low temperature, Can. J. Microbiol., 2014, vol. 60, no. 5, pp. 327–331.
Mangwani, N., Kumari, S., Shukla, SK., Rao, T.S., and Das, S., Phenotyping switching in biofilm-forming bacteria in Paenibacillus lautus, Curr. Microbiol., 2014., vol. 68, no. 5, pp. 648–656.
Ochsner, U.A., Vasil, M.L., Alsabbagh, E., Parvatiyar, K., and Hassett, D.J., Role of the Pseudomonas aeruginosa oxyR-recG operon in oxidative stress defense and DNA repair: OxyR-dependent regulation of katB-ankB, ahpB, andahpC-ahpF, J. Bacteriol., 2000, vol. 182, pp. 4533–4544.
Padan, E., Bibi, E., Ito, M., and Krulwich, T.A., Alkaline pH homeostasis in bacteria: new insights, Biochem. Biophys. Acta, 2005, vol. 30, pp. 67–88.
Pfaffl, M.W., A new mathematical model for relative quantification in real-time RT-PCR, Nucleic Acids Res., 2001, vol. 29, no. 9, pp. e45.
Romanenko, L.A., Uchino, M., Falsen, E., Frolova, G.M., Zhukova, N.V., and Mikhailov, V.V., Pseudomonas pachastrellae sp. nov., isolated from a marine sponge, Int. J. Syst. Evol. Microbiol., 2005, vol. 55, pp. 919–924.
Rupert, C.S., Photoenzymatic repair of ultraviolet damage in DNA, J. Gen. Physiol., 1962, vol. 45, no. 4, pp. 703–724.
Selby, C.P. and Sancar, A., A crytochrome/photolyase class of enzymes with singlestranded DNA-specific photolyase activity, Proc. Natl. Acad. Sci. U. S. A., 2006, vol. 103, no. 47, pp. 17696–17700.
Sinha, R.P. and Häder, D.P., UV-induced DNA damage and repair: a review, Photo. Chem. Photobiol. Sci., 2002, vol. 1, no. 4, pp. 225–236.
Solanky, D. and Haydel, S.E., Adaptation of the neutral bacterial comet assay to assess antimicrobial-mediated DNA double-strand breaks in Escherichia coli, J. Microbiol. Methods., 2012, vol. 91, no. 2, pp. 257–261.
Stanley, S.O. and Morita, R.Y., Salinity effect on the maximal growth temperature of some bacteria isolated from marine environments, J. Bacteriol., 1968, vol. 95, no. 1, pp. 169–173.
Thompson, C.L. and Sancar, A., Photolyase/cryptochrome blue-light photoreceptors use photon energy to repair DNA and reset the circadian clock, Oncogene, 2002, vol. 21, no. 58, pp. 9043–9056.
Zenoff, V.F., Sineriz, F., and Farias, M.E., Diverse response to UV-B radiation and repair mechanisms of bacteria isolated from high-altitude aquatic organisms, Appl. Environ. Microbiol., 2006, vol. 72, no. 12, pp. 7857–7863.
Žgur-Bertok, D., DNA Damage Repair and Bacterial Pathogens, PLoS Pathog., 2013, vol. 9 no. 11, pp. e1003711.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Das, S., Ganeriwal, S., Mangwani, N. et al. Survival and expression of DNA repair genes in marine bacteria Pseudomonas pseudoalcaligenes NP103 and P. aeruginosa N6P6 in response to environmental stressors. Microbiology 84, 644–653 (2015). https://doi.org/10.1134/S0026261715050057
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026261715050057