Abstract
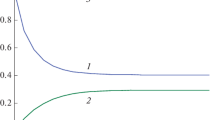
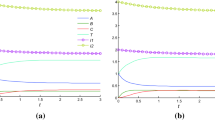
A method has been developed for calculating the exact autonomous kinetic invariants of multistage linear chemical reactions occurring in a gradientless isothermal reactor via any number of elementary stages and involving an arbitrary number of reagents within the framework of mass-action law. These invariants are functions that remain strictly constant throughout the reaction (i.e., are time-independent), although they depend on the nonequilibrium reagent concentrations measured in two experiments with different initial conditions (dual experiments). For the dynamic models of linear reactions, one can always find general and particular analytical solutions expressed explicitly in terms of the initial conditions. The idea of the method is based on choosing the initial conditions that allow the nonequilibrium reagent concentrations to be expressed in terms of constant kinetic parameters (stage rate and flow rate constants). An applicability criterion of the method was formulated. The number of different invariants was shown to be equal to the number of reagents. The relations obtained in this study were used to investigate the relaxation properties of multistage linear reactions occurring in closed and open isothermal systems. The invariant curves found for these reactions were compared with the concentration curves measured in two nonequilibrium experiments throughout the transition process. For these reactions, the time dependences of the invariants remained strictly constant, while the reagent concentrations continuously changed (including nonmonotonously) during the reaction. The results give new insight into the relaxation of linear chemical reactions and can be used to solve the inverse problems of chemical kinetics under the conditions of an isothermal perfect-mixing reactor.



Similar content being viewed by others
REFERENCES
Gorban', A.N., Bykov, V.I., and Yablonskii, G.S., Ocherki o khimicheskoi relaksatsii (Essays on Chemical Relaxation), Novosibirsk: Nauka, 1986.
Kondepudi, D. and Prigozhin, I., Sovremennaya termodinamika. Ot teplovykh dvigatelei do dissipativnykh struktur (Modern Thermodynamics. From Heat Engines to Dissipative Structures), Moscow: Mir, 2002.
Kol’tsov, N.I., Fedotov, V.Kh., and Alekseev, B.V., Kinet. Katal., 1995, vol. 36, no. 1, p. 51.
Kozhevnikov, I.V., Alekseev, B.V., and Kol’tsov, N.I., Kinet. Catal., 1998, vol. 39, no. 6, p. 839.
Alekseev, B.V. and Kol’tsov, N.I., Kinet. Catal., 2002, vol. 43, no. 1, p. 34.
Yablonsky, G., Constales, D., and Marin, G.B., Chem. Eng. Sci., 2011, vol. 66, no. 1, p. 111.
Yablonsky, G.S., Gorban, A.N., and Constales, D., Europhys. Lett., 2011, vol. 93, no. 2, p. 20004.
Constales, D., Yablonsky, G.S., and Marin, G.B., Chem. Eng. Sci., 2012, vol. 73, p. 20.
Constales, D., Yablonsky, G.S., and Marin, G.B., Comp. Math. Appl., 2013, vol. 65, p. 1614.
Yablonsky, G.S., Theor. Found. Chem. Eng., 2014, vol. 48, no. 5, p. 608.
Yablonsky, G., Constales, D., and Marin, G.B., Adv. Chem. Phys., 2014, vol. 157, p. 69.
Branco-Pinto, D., Yablonsky, G., Marin, G., and Constales, D., Entropy, 2015, vol. 17, p. 6783.
Branco, P.D., Yablonsky, G., Marin, G.B., and Constales, D., Chem. Eng. Sci., 2017, vol. 158, p. 370.
Peng, B., Yablonsky, G.S., Constales, D., and Marin, G.B., Chem. Eng. Sci., 2018, vol. 191, p. 262.
Branco, P.D., Yablonsky, G., Marin, G.B., and Constales, D., Chem. Eng. Sci., 2018, vol. 184, p. 25.
Yablonsky, G.S., Branco, P.D., Marin, G.B., and Constales, D., Chem. Eng. Sci., 2019, vol. 196, p. 384.
Fedotov, V.Kh. and Kol’tsov, N.I., Izv. Vyssh. Uchebn. Zaved.,Khim. Khim. Tekhnol., 2016, vol. 59, no. 5, p. 72.
Fedotov, V.Kh., Kol’tsov, N.I., and Kos’yanov, P.M., Vestn. Tekhnol.Un-ta, 2018, vol. 21, no. 12, p. 181.
Fedotov, V.Kh. and Kol’tsov, N.I., Vestn. Tekhnol.Un-ta, 2019, vol. 22, no. 1, p. 122.
Fedotov, V.Kh. and Kol’tsov, N.I., Khim. Fiz., 2019, vol. 38, no. 4, p. 23.
Zel’dovich, Ya.B., Zh. Fiz. Khim., 1938, vol. 11, no. 5, p. 685.
Korn, G. and Korn, T., Spravochnik po matematike (Mathematics Handbook), Moscow: Nauka, 1978.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by L. Smolina
Abbreviations: DEM—dual-experiments method, MEM—multi-experiments method, ODE—ordinary differential equations, CE—characteristic equation, CL—conservation law, mol. fr.— mole fraction, ICs—initial conditions.
Rights and permissions
About this article
Cite this article
Fedotov, V.K., Kol’tsov, N.I. Autonomous Kinetic Invariants of Linear Chemical Reactions. Kinet Catal 60, 776–782 (2019). https://doi.org/10.1134/S002315841906003X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S002315841906003X