Abstract
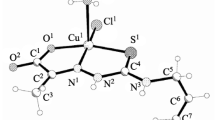
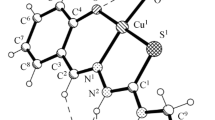
The crystal structures and biological properties of [N′-(2-oxidobenzylidene)-N-(prop-2-en-1-yl)-carbamohydrazonothioato(2-)](1,10-phenanthroline)copper hemihydrate [Cu(1,10-Phen)(L)]·0.5H2O (I) and [N′-(2-oxidobenzylidene)-N-(prop-2-en-1-yl)-carbamohydrazonothioato(2-)](2,2′-bipyridine)copper hemihydrate [Cu(2,2′-BPy)(L)]·0.5H2O (II), where Н2L is 2-(2-hydroxybenzylidene)-N-(prop-2-en-1-yl)hydrazinecarbothioamide, are determined. The asymmetric unit of the unit cell in the crystal structures of I and II contains a copper complex with bidentate amine and a ligand coordinated by the azomethine nitrogen atom, the deprotonated phenolic oxygen atom, and the sulfur atom in the thiol form. The coordination polyhedron of the copper atom in compounds I and II is a distorted tetragonal pyramid. Obtained coordination compounds I and II exhibit antimicrobial and antifungal activities and have minimum inhibitory concentration and bactericidal concentration values in a range of 1.5-500 µg/mL. The study of the antioxidant activity shows that compounds I and II are less active than uncoordinated thiosemicarbazone H2L, but more active than trolox used in medical practice.






Similar content being viewed by others
REFERENCES
Y. Yu, D. S. Kalinowski, Z. Kovacevic, A. Siafakas, P. Jansson, C. Stefani, D. Lovejoy, P. Sharpe, P. Bernhardt, and D. Richardson. J. Med. Chem., 2009, 52, 5271-5294. https://doi.org/10.1021/jm900552r
D. Klayman, J. Scovill, J. Bartosevich, and C. Mason. J. Med. Chem., 1979, 22, 1367. https://doi.org/10.1021/jm00197a017
M. S. Villis, S. A. Monaghan, M. L. Miller, R. W. McKenna, W. D. Perkins, B. S. Levinson, V. Bhushan, and S. H. Kroft. Am. J. Clin. Pathol., 2005, 123(1), 125-131. https://doi.org/10.1309/V6GVYW2QTYD5C5PJ
S. Archana and J. Ezhilarasi Rosaline. Int. J. Environ. Res. Chem. Environ., 2012, 2(4), 130-148.
J. Patole, S. Padhye, S. Padhye, C. J. Newton, C. Anson, and A. K. Powell. Indian J. Chem., Sect. A: Inorg., Bio-inorg., Phys., Theor. Anal. Chem., 2004, 43, 1654-1658.
S. Orysyk, G. Repich, V. Bon, V. Dyakonenko, V. Orysyk, Y. Zborovskii, and M. Vovk. Inorg. Chim. Acta, 2014, 423, 496. https://doi.org/10.1016/j.ica.2014.08.056
E. Pahontu, V. Fala, A. Gulea, D. Poirier, V. Tapcov, and T. Rosu. Molecules, 2013, 18, 8812. https://doi.org/10.3390/molecules18088812
P. Bindu, M. R. P. Kurup, and T. R. Satyakeerty. Polyhedron, 1998, 18, 321. https://doi.org/10.1016/s0277-5387(98)00166-1
V. Prisakar, V. Tsapkov, S. Buracheeva, M. Byrke, and A. Gulea. Pharm. Chem. J., 2005, 39, 313. https://doi.org/10.1007/s11094-005-0142-8
A. Gulea, V. Graur, I. Ulchina, P. Bourosh, V. Smaglii, O. Garbuz, and V. Tsapkov. Russ. J. Gen. Chem., 2021, 91(1), 98-107. https://doi.org/10.1134/S1070363221010114
S. I. Orysyk, V. V. Bon, O. O. Zholob, V. I. Pekhnyo, V. V. Orysyk, Y. L. Zborovskii, and M. V. Vovk. Polyhedron, 2013, 51, 211-221. https://doi.org/10.1016/j.poly.2012.12.021
J. Fries and H. Getrost. Organic Reagents for Trace Analysis. Darmstadt: E. Merck, 1977.
CrysAlisPro, 1.171.33.52. Oxford Diffraction, 2009.
G. M. Sheldrick. Acta Crystallogr., Sect. A, 2008, 64, 112. https://doi.org/10.1107/S0108767307043930
A. L. Spek. J. Appl. Crystallogr., 2003, 36, 7. https://doi.org/10.1107/S0021889802022112
A. Gulea, D. Poirier, J. Roy, V. Stavila, I. Bulimestru, V. Tapcov, and L Popovschi. J. Enzyme Inhib. Med. Chem., 2008, 23, 806. https://doi.org/10.1080/14756360701743002
G. Balan, O. Burduniuc, I. Usataia, V. Graur, Yu. Chumakov, P. Petrenko, V. Gudumac, A. Gulea, and E. Pahontu. Appl. Organomet. Chem., 2020, 34, e5423. https://doi.org/10.1002/aoc.5423
Funding
The work was performed within State Programs of the Republic of Moldova (projects 20.80009.5007.10 and 20.80009.5007.15).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interests.
Additional information
Russian Text © The Author(s), 2022, published in Zhurnal Strukturnoi Khimii, 2022, Vol. 63, No. 6, pp. 771-779.https://doi.org/10.26902/JSC_id92728
Rights and permissions
About this article
Cite this article
Chumakov, Y.M., Graur, V.O., Ulchina, Y.I. et al. CRYSTAL STRUCTURES OF [N′-(2- OXIDOBENZYLIDENE)-N-(PROP-2-EN-1-YL)- CARBAMOHYDRAZONOTHIOATO(2-)](1,10- PHENANTHROLINE)COPPER AND [N′-(2- OXIDOBENZYLIDENE)-N-(PROP-2-EN-1-YL)- CARBAMOHYDRAZONOTHIOATO(2-)](2,2′- BIPYRIDINE)COPPER HEMIHYDRATES. J Struct Chem 63, 905–913 (2022). https://doi.org/10.1134/S0022476622060075
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476622060075