Abstract
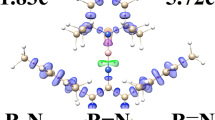
Due to the importance of structural properties of annulene in physical and inorganic chemistry, the trajectory of the NBO to MO evolution can be written as natural atomic orbitals (NAO) → natural hybrid orbital (NHO) → natural bond orbital (NBO) → natural semi-localized MO (NLMO) → MO. The electron density distribution in this [n]-annulene series (both ions and molecules) (n = 8, 10, 12, 14) is investigated by NMR, NBO, ELF, FLU, and PDI analyses. The (4n+2)π and also 4nπ systems (Hückel’s rule) on variants of those compounds via the localized orbital localization (LOL) and electron localized function (ELF) are discussed, and a diatropic ring current (aromatic) is also distinguished for some other paratropic currents (anti-aromatic). The NHO direction and bond bending deviations from the line of nuclear centers are exhibited for understanding the situation of π and σ orbitals. In this work, for each NAO function, core, valence, or Rydberg, the orbital occupancy and the orbital energies are discussed. In addition, nucleus independent chemical shifts (NICSs) and statistical nucleus chemical shifts (S-NICSs) confirm the aromaticity and anti-aromaticity amounts in those rings.
Similar content being viewed by others
References
P. Willstätter, E. Waser, and R. Willstätter. Ber. Dtsch. Chem. Ges., 1911, 44(3), 3423–3445.
J. F. M. Oth. Pure Appl. Chem., 1971, 25, 573–622.
J. I. Wu, I. Fernández, Y. Mo, and P. V. R. Schleyer. J. Chem. Theory Comput., 2012, 8, 1280–1287.
R. Naor and Z. Luz. J. Chem. Phys., 1982, 76, 5662–5664.
J. L. Andrés, O. Castaño, A. Morreale, R. Palmeiro, and R. Gomperts. J. Chem. Phys., 1998, 108, 203–207.
T. Nishinaga, T. Ohmae, and M. Iyoda. Symmetry, 2010, 2, 76–97.
C. Gellini and P. R. Salvi. Symmetry, 2010, 2, 1846–1924.
P. G. Wenthold, D. A. Hrovat, W. T. Borden, and W. C. Lineberger. Science, 1996, 272, 1456–1459.
A. Schild and B. Paulus. J. Comput. Chem., 2013, 34, 1393–1397.
D. A. Hrovat and W. T. Borden. J. Am. Chem. Soc., 1992, 114, 5879–5881.
C. D. Stevenson, E. C. Brown, D. A. Hrovat, and W. T. Borden. J. Am. Chem. Soc., 1998, 120, 8864–8867.
A. Schild and B. J. Paulus. J. Comput. Chem., 2013, 34, 1393–1397.
T. Yoshida and C. Tokizaki. Chem. Phys. Lett., 2015, 634, 134–139.
E. Steiner and P. W. Fowler. J. Phys. Chem. A, 2001, 105, 9553–9562.
E. Steiner, P. W. Fowler, and R. W. A. Havenith. J. Phys. Chem. A, 2002, 106, 7048–7056.
F. London. J. Phys. Radium., 1937, 8, 397–409.
L. Pauling. J. Chem. Phys., 1936, 4, 673–677.
J. A. Pople. J. Chem. Phys., 1956, 24, 1111.
P. von R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, and N. J. R. van Eikema Hommes. J. Am. Chem. Soc., 1996, 118, 6317–6318.
A. Soncini, P. W. Fowler, and L. W. Jenneskens. Phys. Chem. Chem. Phys., 2004, 6, 277–284.
F. A. L. Anet and D. J. O’Leary. Concepts Magn. Reson., 1992, 4, 35.
U. Haeberlen. In: Advances in Magnetic Resonance. Suppl. 1. Academic Press: New York, 1976.
M. Mehring. High Resolution NMR Spectroscopy in Solids, 2nd. Ed. Springer Verlag: Berlin, 1983.
NMR Basic Principles and Progress./Eds. P. Diehl, E. Fluck, and R. Kosfeld. Springer Verlag: Berlin, 1978, 15.
R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, P. Granger, R. E. Hoffman, and K. W. Zilm. Ann. Magn. Reson., 2008, 7, 1.
J. Herzfeld and A. E. Berger. J. Chem. Phys., 1980, 73, 6021.
R. F. W. Bader. Atoms in Molecule: A quantum Theory. Oxford Univ. Press: Oxford, 1990.
A. D. Becke and K. E. Edgecombe. J. Chem. Phys., 1990, 92, 5397.
A. Savin, O. Jepsen, J. Flad, O. K. Andersen, H. Preuss, and H. G. von Schnering. Angew. Chem., Int. Ed. Engl., 1994, 31(2), 187.
A. D. Becke. J. Mol. Struct.: THEOCHEM, 2000, 527, 51.
H. Jacobsen. Can. J. Chem., 2008, 86(7), 695–702.
W. Kohn and L. J. Sham. J. Phys. Rev., 1965, 140, A1133–1138.
J. P. Perdew, K. Burke, and Ernzerhof. Phys. Rev. Lett., 1996, 77, 3865–3868.
T. Lu and F. Chen. J. Mol. Graph. Model., 2012, 38, 314–323.
T. Lu and F. Chen. J. Comp. Chem., 2012, 33, 580–592.
B. H. Besler, K. M. Merz, and P. A. Kollman. J. Comp. Chem., 1990, 11, 431–439.
L. E. Chirlian and M. M. Francl. J. Comp. Chem., 1987, 8, 894–905.
F. Martin and H. Zipse. J. Comp. Chem., 2005, 26, 97–105.
M. Monajjemi, V. S. Lee, M. Khaleghian, B. Honarparvar, and F. Mollaamin. J. Phys. Chem. C, 2010, 114, 15315.
M. Monajjemi and M. Khaleghian. J. Clust. Sci., 2011, 22, 673–692.
M. Monajjemi. J. Struct. Chem., 2012, 23, 551.
M. Monajjemi and J. E. Boggs. J. Phys. Chem. A.2013, 117, 1670–1684.
M. Monajjemi, W. J. Robert, and J. E. Boggs. Chem. Phys., 2014, 433, 1–11.
M. Monajjemi, M. Khosravi, B. Honarparvar, and F. Mollaamin. Int. J. Quantum Chem., 2011, 111, 2771–2777.
M. Monajjemi. Theor. Chem. Acc., 2015, 134(77), 1–22.
M. Monajjemi. J. Mol. Model., 2014, 20, 2507.
A. J. Bridgeman. Polyhedron, 1998, 17, 2279–2288.
M. Monajjemi and N. T. Mohammadian. J. Comput. Theor. Nanosci., 2015, 12, 4895–4914.
A. A. Frost and B. A. Musulin. J. Chem. Phys., 1953, 21, 572.
A. Matsuura and K. Komatsu. J. Am. Chem. Soc., 2001, 123, 1768–1769.
E. W. Stout and P. Politzer. Theor. Chim. Acta, 1968, 12(5), 379–386.
Acknowledgments
Our common idea and preliminary discussion of this work refer to the duration of my sabbatical collaborating with professor James E. Boggs (who passed away) in the Institute for Theoretical Chemistry, Department of Chemistry and Biochemistry, The University of Texas at Austin, Austin, Texas, United States that reminds me his memory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare that they have no conflict of interests.
Text © The Author(s), 2020, published in Zhurnal Strukturnoi Khimii, 2020, Vol. 61, No. 2, pp. 221–238.
Supplementary materials
10947_2020_1351_MOESM1_ESM.pdf
SUPPLEMENTARY MATERIALS TO: MOLECULAR STRUCTURAL PROPERTIES OF [n]-ANNULENE (n = 8, 10, 12, 14) AND ITS BORON NITRIDE DERIVATIVES: ANALYSIS OF NMR, NBO, ELF AND PDI
Rights and permissions
About this article
Cite this article
Monajjemi, M., Mollaamin, F. Molecular Structural Properties of [n]-Annulene (n = 8, 10, 12, 14) and its Boron Nitride Derivatives: Analysis of NMR, NBO, ELF and PDI. J Struct Chem 61, 207–224 (2020). https://doi.org/10.1134/S0022476620020055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476620020055