Abstract
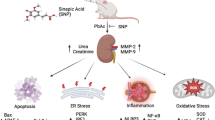
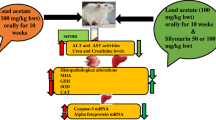
The review addresses the mechanisms of lead acetate toxic effects on the cardiovascular and nervous systems, as well as visceral organs. Experimental and clinical studies conducted in recent years have shown an important pathogenetic role of oxidative stress in the dysfunction of the vascular endothelium, leading to the development of pathology of internal organs, especially the kidneys and liver. Activation of free-radical processes and disruption of cellular bioenergetics are accompanied by a decrease in the production of nitric oxide, a major vasodilator, leading to endothelial dysfunction, increased vascular tone, and eventually to systemic vasoconstriction. On the other hand, reactive oxygen species and lipid peroxidation products stimulate the synthesis of pro-inflammatory cytokines and pro-apoptotic proteins, causing intracellular inflammatory processes in the visceral organs, DNA damage, cell apoptosis, as well as persistent renal and hepatic dysfunction. In this regard, there have been developed various pharmaceuticals with antioxidant and anti-inflammatory properties, and those able to improve cellular bioenergetics of the visceral organs. Among them are such phyto-derivatives as Moringa oleifera extract, curcumin, vitamin C, vitamin C–curcumin combination, as well as analogs of endogenous antioxidants, L-carnitine and coenzyme Q10. All tissues contain significant amounts of ubiquinone, which is essential for cellular functions, including electron and proton transport in the mitochondrial respiratory chain and antioxidant defense.

Similar content being viewed by others
REFERENCES
de Souza ID, de Andrade AS, Dalmolin RJS (2018) Lead-interacting proteins and their implication in lead poisoning. Crit Rev Toxicol 48(5):375–386. https://doi.org/10.1080/10408444.2018.1429387
Ericson B, Gabelaia L, Keith J, Kashibadze T, Beraia N, Sturua L, Kazzi Z (2020) Elevated Levels of Lead (Pb) Identified in Georgian Spices. Ann Glob Health 86(1):124. https://doi.org/10.5334/aogh.3044
Mani MS, Kabekkodu SP, Joshi MB, Dsouza HS (2019) Ecogenetics of lead toxicity and its influence on risk assessment. Hum Exp Toxicol 38(9):1031–1059. https://doi.org/10.1177/0960327119851253
Obeng-Gyasi E (2019) Sources of lead exposure in various countries. Rev Environ Health 34(1):25–34. https://doi.org/10.1515/reveh-2018-0037
Boskabady M, Marefati N, Farkhondeh T, Farzaneh Sh, Farshbaf A, Boskabady MH (2018) The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environment Int 120:404–420. https://doi.org/10.1016/j.envint.2018.08.013
Caito S, Lopes ACBA, Paoliello MB, Aschner M (2017) Toxicology of Lead and Its Damage to Mammalian Organs. In: Sigel A, Sigel H, Sigel RKO (eds) Lead: Its Effects on Environment and Health. De Gruyter, Berlin, pp 501–534. https://doi.org/10.1515/9783110434330-016
Minigalieva IA, Shtin TN, Makeyev OH, Panov VG, Privalova LI, Gurvic VB, Sutunkova MP, Bushueva TV, Sakhautdinova RR, Klinova SV, Solovyeva SN, Chernyshov IN, Shuman EA, Korotkov AA, Katsnelson BA (2020) Some outcomes and a hypothetical mechanism of combined lead and benzo(a)pyrene intoxication, and its alleviation with a complex of bioprotectors. Toxicol Rep 12(7):986–994. https://doi.org/10.1016/j.toxrep.2020.08.004
Mani MS, Joshi MB, Shetty RR, DSouza VL, Swathi M, Kabekkodu SP, Dsouza HS (2020) Lead exposure induces metabolic reprogramming in rat models. Toxicol Lett 15;335:11–27. https://doi.org/10.1016/j.toxlet.2020.09.010
Kuz’mina LP, Hotuleva A, Bezrukavnikova LM, Sorkina NS, Cidil’kovskaya YeS (2018) The use of modern clinical and laboratory methods of research in the conduct of biological monitoring of the impact of lead on the body of workers at a lead processing enterprise. Public Health and Habitat 7:(304). https://cyberleninka.ru/article/n/ispolzovanie-sovremennyh-kliniko-laboratornyh-metodov-issledovaniya-pri-provedenii-biologicheskogo-monitoringa-vozdeystviya-svintsa
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191(7):419. https://doi.org/10.1007/s10661-019-7528-7
Al Osman M, Yang F, Massey IY (2019) Exposure routes and health effects of heavy metals on children. Biometals 32(4):563–573. https://doi.org/10.1007/s10534-019-00193-5
Evens A, Hryhorczuk D, Lanphear BP, Rankin К, Lewis DA, Forst L, Rosenberg D (2015) The impact of low-level lead toxicity on school performance among children in the Chicago Public Schools: a population-based retrospective cohort study. Environ Health 14: 21. https://doi.org/10.1186/s12940-015-0008-9
Fan Y, Zhao X, Yu J, Xie J, Li C, Liu D, Tang C, Wang C (2020) Lead-induced oxidative damage in rats/mice: A meta-analysis. J Trace Elem Med Biol 58:126443. https://doi.org/10.1016/j.jtemb.2019.126443
Bakulski KM, Seo YA, Hickman RC, Brandt D, Vadari HS, Hu H, Park SK (2020) Heavy Metals Exposure and Alzheimer’s Disease and Related Dementias. J Alzheimers Dis 76(4):1215–1242. https://doi.org/10.3233/JAD-200282
Abdel-Daim MM, Alkahtani S, Almeer R, Albasher G (2020) Alleviation of lead acetate-induced nephrotoxicity by Moringa oleifera extract in rats: highlighting the antioxidant, anti-inflammatory, and anti-apoptotic activities. Environ Sci Pollut Res Int 27(27):33723–33731. https://doi.org/10.1007/s11356-020-09643-x
Alhusaini AM, Faddah LM, Hasan IH, Jarallah SJ, Alghamdi SH, Alhadab NM, Badr A, Elorabi N, Zakaria E, Al-Anazi A (2019) Vitamin C and Turmeric Attenuate Bax and Bcl-2 Proteins’ Expressions and DNA Damage in Lead Acetate-Induced Liver Injury. Dose Response 17(4):1559325819885782. https://doi.org/10.1177/1559325819885782
Li Y, Lv H, Xue C, Dong N, Bi C, Shan A (2021) Plant Polyphenols: Potential Antidotes for Lead Exposure. Biol Trace Elem Res 199(10):3960–3976. https://doi.org/10.1007/s12011-020-02498-w
Gundacker C, Forsthuber M, Szigeti T, Kakucs R, Mustieles V, Fernandez MF, Bengtsen E, Vogel U, Hougaard KS, Saber AT (2021) Lead (Pb) and neurodevelopment: A review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int J Hyg Environ Health 238:113855. https://doi.org/10.1016/j.ijheh.2021.113855
Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, Chlubek D, Baranowska-Bosiacka I (2018) Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int J Mol Sci 20;19(6):1813. https://doi.org/10.3390/ijms19061813
Dzugkoev SG, Dzugkoeva FS, Margieva OI, Mozhaeva IV (2022) Analysis of the mechanisms of violation of the functional parameters of internal organs in saturnism in the experiment in rats. Bull Exp Biol Med 173(2): 177–181. https://doi.org/10.47056/0365-9615-2022-173-2-177-181
Friedrich T, Tavraz NN, Junghans C (2016) ATP1A2 Mutations in Migraine: Seeing through the Facets of an Ion Pump onto the Neurobiology of Disease. Front Physiol 7:239. https://doi.org/10.3389/fphys.2016.00239
Yücebilgiç G, Bilgin R, Tamer L, Tükel S (2003) Effects of lead on Na(+)–K(+)ATPase and Ca(+2)ATPase activities and lipid peroxidation in blood of workers. Int J Toxicol 22(2):95–97. https://doi.org/10.1080/10915810305096
Nazima B, Manoharan V, Miltonprabu S (2016) Oxidative stress induced by cadmium in the plasma, erythrocytes and lymphocytes of rats: Attenuation by grape seed proanthocyanidins. Hum Exp Toxicol 35(4):428–447. https://doi.org/10.1177/0960327115591376
Kloza M, Baranowska-Kuczko M, Toczek M, Kusaczuk M, Sadowska O, Kasacka I, Kozłowska H (2019) Modulation of Cardiovascular Function in Primary Hypertension in Rat by SKA-31, an Activator of K Ca 2.x and K Ca 3.1 Channels. Int J Mol Sci 20(17):4118. https://doi.org/10.3390/ijms20174118
Al-Megrin WA, Soliman D, Kassab RB, Metwally DM, Ahmed E Abdel Moneim, El-Khadragy MF (2020) Coenzyme Q10 Activates the Antioxidant Machinery and Inhibits the Inflammatory and Apoptotic Cascades Against Lead Acetate-Induced Renal Injury in Rats. Front Physiol 7:11:64. https://doi.org/10.3389/fphys.2020.00064
Satarug S, C Gobe G, A Vesey D, Phelps KR (2020) Cadmium and Lead Exposure, Nephrotoxicity, and Mortality. Toxics 8(4):86. https://doi.org/10.3390/toxics8040086
Abdelhamid FM, Mahgoub HA, Ateya AI (2020) Ameliorative effect of curcumin against lead acetate-induced hemato-biochemical alterations, hepatotoxicity, and testicular oxidative damage in rats. Environ Sci Pollut Res Int 1:10950–10965. https://doi.org/10.1007/s11356-020-07718-3
Okesola MA, Ajiboye BO, Oyinloye BE, Ojo OA (2019) Effect of Zingiber officinale on some biochemical parameters and cytogenic analysis in lead-induced toxicity in experimental rats. Toxicol Mech Methods 29(4):255–262. https://doi.org/10.1080/15376516.2018.1558321
Fiorim J, Simões MR, de Azevedo BF, Ribeiro RF Junior, Dos Santos L, Padilha AS, Vassallo DV (2020) Increased endothelial nitric oxide production after low level lead exposure in rats involves activation of angiotensin II receptors and PI3K/Akt pathway. Toxicology 443:152557. https://doi.org/10.1016/j.tox.2020.152557
Abdel-Zaher AO, Abd-Ellatief RB, Aboulhagag NA, Farghaly HSM, Al-Wasei FMM (2019) The interrelationship between gasotransmitters and lead-induced renal toxicity in rats. Toxicol Lett 310:39–50. https://doi.org/10.1016/j.toxlet.2019.04.012
Apyhtina EA, Kocuba AV, Korkach JuP, Andrusishina IN, Lampeka EG (2007) Vasotoxic effect of lead: the role of disturbances in the metabolism of endogenous nitric oxide. Ukr J Occupat Med 3(11):56–62. https://doi.org/10.33573/ujoh2007.03.056
Yakimova NL, Sosedova LM, Vokina VA, Titov EA, Novikov MA (2017) Experimental lead intoxication symptoms with hyperglycemia state. Med Tr Prom Eko 2:54–56.
El-Sherbini ES, El-Sayed G, El Shotory R, Gheith N, Abou-Alsoud M, Harakeh SM, Karrouf GI (2017) Ameliorative effects of l-carnitine on rats raised on a diet supplemented with lead acetate. Saudi J Biol Sci 24(6):1410–1417. https://doi.org/10.1016/j.sjbs.2016.08.010
Mazandaran AA, Khodarahmi P (2021) The protective role of Coenzyme Q10 in metallothionein-3 expression in liver and kidney upon rats’ exposure to lead acetate. Mol Biol Rep 48(4):3107–3115. https://doi.org/10.1007/s11033-021-06311-2
Abdallah GM, El-Sayed el-SM, Abo-Salem OM (2010) Effect of lead toxicity on coenzyme Q levels in rat tissues. Food Chem Toxicol 48(6):1753–1756. https://doi.org/10.1016/j.fct.2010.04.006
Rocha A, Trujillo KA (2019) Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 73:58–80. https://doi.org/10.1016/j.neuro.2019.02.021
Yousef AOS, Fahad AA, Moneim AEA, Metwally DM, El-Khadragy MF, Kassab RB (2019) The Neuroprotective Role of Coenzyme Q10 Against Lead Acetate-Induced Neurotoxicity Is Mediated by Antioxidant, Anti-Inflammatory and Anti-Apoptotic Activities. Int J Environ Res Public Health 16(16):2895. https://doi.org/10.3390/ijerph16162895
Hargreaves I, Heaton RA, Mantle D (2020) Disorders of Human Coenzyme Q10 Metabolism: An Overview. Int J Mol Sci 21(18):6695. https://doi.org/10.3390/ijms21186695
Goroshko OA, Krasnykh LM, Kukes VG, Zozina VI (2019) Significance of the redox status of coenzyme Q10 as a biomarker of oxidative stress. Bull Scientif Center for Expertise of Med Applicat 9(3):146–152. https://cyberleninka.ru/article/n/znachenie-redoks-statusa-koenzima-q10-kak-biomarkera-okislitelnogo-stressa
Pradedova EV, Nimaeva OD, Salyaev RK (2014) Redox enzymes of Red Beetroot vacuoles (Beta vulgaris L.). J Stress Physiol Biochem 4: 98–117. https://cyberleninka.ru/article/n/redox-enzymes-of-red-beetroot-vacuoles-beta-vulgaris-l
Funding
This review was written within the state budget-funded scientific research assignment to the Institute of Biomedical Research, Branch of the Vladikavkaz Scientific Center of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Contributions
Conceptualization and editing the manuscript (S.G.D.); literature data collection, analysis of publications, writing and editing the manuscript (S.G.D., F.S.D., O.I.M.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have neither evident nor potential conflict of interest related to the publication of this review article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2022, Vol. 108, No. 5, pp. 626–635https://doi.org/10.31857/S0869813922050028.
Rights and permissions
About this article
Cite this article
Dzugkoev, S.G., Dzugkoeva, F.S. & Margieva, O.I. Mechanisms of Lead Toxicity and Their Pathogenetic Correction. J Evol Biochem Phys 58, 807–814 (2022). https://doi.org/10.1134/S0022093022030140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022030140