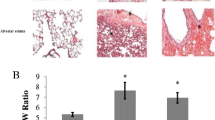
Abstract
Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are the major proinflammatory cytokines. Cytokine receptors are expressed in the brainstem areas involved in respiratory control, as well as in the carotid bodies that control the O2 balance in arterial blood. We hypothesized that circulating proinflammatory cytokines are able to affect lung ventilation and modulate the respiratory response to hypoxia by activating NO-dependent pathways. The aim of our study was to compare the respiratory effects of IL-1β and TNF-α before and after pretreatment with L-NAME, a non-selective inhibitor of NO synthase (NOS). The ventilatory response to hypoxia was measured in anesthetized Wistar rats using the rebreathing method before and after intravenous administration of IL-1β (2 µg/kg) and TNF-α (40 µg/kg). It was found that an increase in the systemic level of proinflammatory cytokines increases lung ventilation under normoxia, while reducing respiratory sensitivity to hypoxia. Pretreatment with L-NAME (intraperitoneal administration) reduced these respiratory effects of both IL-1β and TNF-α. We believe that the activation of NOS pathways and an increase in NO synthesis, when cytokines interact with the corresponding receptors, mediate the respiratory effects of proinflammatory cytokines and underlies the effect of inflammation on respiratory function.




Similar content being viewed by others
REFERENCES
Wang X, Wang BR, Duan X L, Zhang P, Ding YQ, Jia Y, Jiao XY, Ju G (2002) Strong expression of interleukin-1 receptor types I in the rat carotid body. J Histochem Cytochem 50 (12):1677–1684. https://doi.org/10.1177/002215540205001213
Wang X, Zhang XJ, Xu Z, Li X, Li G L, Ju G, Wang BR (2006) Morphological evidence for existence of IL-6 receptor alpha in the glomus cells of rat carotid body. Anat Rec A Discov Mol Cell Evol Biol 288 (3):292–296. https://doi.org/10.1002/ar.a.20310
Lam SY, Tipoe GL, Liong EC, Fung ML (2008) Chronic hypoxia upregulates the expression and function of proinflammatory cytokines in the rat carotid body. Histochem Cell Biol 130 (3):549–559. https://doi.org/10.1007/s00418-008-0437-4
Gauda EB, Shirahata M, Masona A, Pichard LE, Kostuk EW, Chavez-Valdeza R (2013) Inflammation in the carotid body during development and its contribution to apnoea of prematurity. Respir Physiol Neurobiol 185 (1):120–131. https://doi.org/10.1016/j.resp.2012.08.005
Vgontzas AN, Papanicolaou DA, Bixler EO (2000) Sleep apnoea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85:1151–1158.
Vernooy JH, Kucukaycan M, Jacobs JA, Chavannes NH, Buurman WA, Dentener MA, Wouters EF (2002) Local and systemic inflammation in patients with chronic obstructive pulmonary disease: soluble tumor necrosis factor receptors are increased in sputum. Am J Respir Crit Care Med 166: 1218–1224.
Bucchioni E, Kharitonov SA, Allegra L, Barnes PJ (2003) High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir Med 97:1299–1302.
Fernández R, González S, Rey S, Cortés PP, Maisey KR, Reyes EP, Larraín C, Zapata P (2008) Lipopolysaccharide-induced carotid body inflammation in cats: functional manifestations, histopathology and involvement of tumour necrosis factor-alpha. Exp Physiol 93 (7):892–907. https://doi.org/10.1113/expphysiol.2008.041152
Zapata P, Larrain C, Reyes P, Fernández R (2011) Immunosensory signaling by carotid body chemoreceptors. Respir Physiol Neurobiol 178 (3):370–374. https://doi.org/10.1016/j.resp.2011.03.025
Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS (2011) Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respir Physiol Neurobiol 178 (3):482–489. https://doi.org/10.1016/j.resp.2011.06.017
Nakamori T, Morimoto A, Murakami N (1993) Effect of a central CRF antagonist on cardiovascular and thermoregulatory responses induced by stress or IL-1β. Am J Physiol 265 (4):834–839.
Watanabe T, Tan N, Saiki Y, Makisumi T, Nakamura S (1996) Possible involvement of glucocorticoids in the modulation of interleukin-1-induced cardiovascular responses in rats. J Physiol 491 (1):231–239. https://doi.org/10.1113/jphysiol.1996.sp021211
Graff GR, Gozal D (1999) Cardiorespiratory responses to interleukin-1beta in adult rats: role of nitric oxide, eicosanoids and glucocorticoids. Arch Physiol Biochem 107 (2):97–112.
Herlenius E (2011) An inflammatory pathway to apnea and autonomic dysregulation. Respir Physiol Neurobiol 178:449–457. https://doi.org/10.1016/j.resp.2011.06.026
Aleksandrova NP, Danilova GA, Aleksandrov VG (2015) Cyclooxygenase pathway in modulation of the ventilatory response to hypercapnia by interleukin-1β in rats. Respir Physiol Neurobiol 209:85–90. https://doi.org/10.1016/j.resp.2014.12.006
Foster SJ, McCormick LM, Ntolosi BA, Campbell D (1993) Production of TNF alpha by LPS-stimulated murine, rat and human blood and its pharmacological modulation. Agents Actions 38:77–79. https://doi.org/10.1007/BF01991143
Preas HL 2nd, Jubran A, Vandivier RW, Reda D, Godin PJ, Banks SM, Tobin MJ, Suffredini AF (2001) Effect of endotoxin on ventilation and breath variability: role of cyclooxygenase pathway. Am J Respir Crit Care Med 164 (4):620–626.
Aleksandrova NP, Danilova GA, Aleksandrov VG (2017) Interleukin-1beta suppresses the ventilatory hypoxic response in rats via prostaglandin dependent pathways. Canad J Physiol Pharmacol 95 (6):681–685. https://doi.org/10.1139/cjpp-2016-0419
Tanaka K, Chiba T (1994) Nitric oxide synthase containing neurons in the carotid body and sinus of the guinea pig. Microscopy Res Techniq J 29(2):90–93. https://doi.org/10.1002/jemt.1070290205
Iturriaga R (2001) Nitric oxide and carotid body chemoreception. Biol Res 34(2):135–139. https://doi.org/10.4067/s0716-97602001000200019
Moya EA, Alcayaga J, Iturriaga R (2012) NO modulation of carotid body chemoreception in health and disease. Respir Physiol Neurobiol 184 (2):158–164. https://doi.org/10.1016/j.resp.2012.03.019
Li HF, Yu J (2009) Airway chemosensitive receptors in vagus nerve perform neuro-immune interaction for lung-brain communication. Adv Exp Med Biol 648:421–426. https://doi.org/10.1007/978-90-481-2259-2_48
Valdés V, Mosqueira M, Rey S (2003) Inhibitory effects of NO on carotid body: contribution of neural and endothelial nitric oxide synthase isoforms. Am J Physiol Lung Cell Mol Physiol 284 (1):57–68.
Funding
This work was supported by the State Program of the Russian Federation (47 SP).
Author information
Authors and Affiliations
Contributions
Basic idea and experimental design (N.P.A., A.A.K.); data collection (A.A.K., G.A.D.); data processing (A.A.K., G.A.D.); writing and editing a manuscript (A.A.K., N.P.A., G.A.D.).
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors assures that they have neither evident nor potential conflict of interest associated with the publication of this article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2021, Vol. 107, No. 11, pp. 1385–1394https://doi.org/10.31857/S0869813921110042.
Rights and permissions
About this article
Cite this article
Klinnikova, A.A., Danilova, G.A. & Aleksandrova, N.P. Role of Nitric Oxide Synthase Pathways in the Effects of Proinflammatory Cytokines on the Respiratory Pattern and Hypoxic Ventilatory Response. J Evol Biochem Phys 57, 1373–1381 (2021). https://doi.org/10.1134/S0022093021060168
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021060168