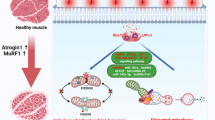
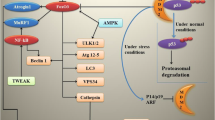
Abstract
Autophagy is an evolutionarily conserved process of degradation of intracellular structures by lysosomal enzymes in specialized compartments such as autophagolysosomes plays a role in many processes, such as differentiation, maintenance of energy homeostasis, and protection of cells in the presence of destructive changes. Autophagy is of particular importance for the functioning of skeletal and cardiac muscles, namely, to maintain the structural and physiological integrity of the sarcomere during muscle contraction, as well as for pathological changes in the muscle fiber. Activation of the autophagy process occurs in response to a variety of stressful stimuli, such as muscle damage during intense exercise, resulting in tissue repair, including through the activation of satellite cells. In this review, autophagy is considered as a protective process, in which several types are distinguished, differing in their mechanisms. The review will cover the molecular basis of the autophagy process, its role in the vital activity and functioning of cells, as well as the therapeutic potential of autophagy activators in the treatment of severe human diseases associated with disorders of skeletal and cardiac muscles. Special attention will be paid to the description of pharmacological drugs that can enhance the activity of autophagy, as well as the mechanisms of their action.

Similar content being viewed by others
Abbreviations
- AA:
-
amino acids
- ROS:
-
reactive oxygen species
- DMD:
-
Duchenne myodystrophy
- AMPK:
-
AMP-activated protein kinase
- Atg :
-
genes associated with autophagy
- CASA:
-
chaperone-assisted selective autophagy
- HSP:
-
heat shock proteins
- mTOR:
-
mammalian target of rapamycin
- ULK1:
-
Unc-51 like autophagy-initiating kinase 1
- LC3:
-
microtubule-associated protein 1 light chain 3
REFERENCES
Condello M, Pellegrini E, Caraglia M, Meschini S (2019) Targeting autophagy to overcome human diseases. Int J Mol Sci 20(3):725. https://doi.org/10.3390/ijms20030725
Kirkin V, Rogov VV (2019) A Diversity of Selective Autophagy Receptors Determines the Specificity of the Autophagy Pathway. Mol Cell 76:268–285. https://doi.org/10.1016/j.molcel.2019.09.005
Wu NN, Tian H, Chen P, Wang D, Ren J, Zhang Y (2019) Physical Exercise and Selective Autophagy: Benefit and Risk on Cardiovascular Health. Cells 8(11):1436. https://doi.org/10.3390/cells8111436
Valenzuela CA, Ponce C, Zuloaga R, González P, Avendaño-Herrera R, Valdés JA, Molina A (2020) Effects of crowding on the three main proteolytic mechanisms of skeletal muscle in rainbow trout (Oncorhynchus mykiss). BMC Vet Res 16:294. https://doi.org/10.1186/s12917-020-02518-w
Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z (2013) Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J 27:4184–4193. https://doi.org/10.1096/fj.13-228486
Jokl EJ, Blanco G (2016) Disrupted autophagy undermines skeletal muscle adaptation and integrity. Mamm Genome 27(11):525–537. https://doi.org/10.1007/s00335-016-9659-2
Bell RAV, Al-Khalaf M, Megeney LA (2016) The beneficial role of proteolysis in skeletal muscle growth and stress adaptation. Skelet Muscle 6:16. https://doi.org/10.1186/s13395-016-0086-6
Jiao J, Demontis F (2017) Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin. Pharmacol 34:1–6. https://doi.org/10.1016/j.coph.2017.03.009
Mammucari C, Rizzuto R (2010) Signaling pathways in mitochondrial dysfunction and aging. Mech Ageing Dev 131:536–543. https://doi.org/10.1016/j.mad.2010.07.003
Lee D, Bareja A, Bartlett D, White J (2019) Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration. Cells 8:183. https://doi.org/10.3390/cells8020183
Dorsch LM, Schuldt M, Knežević D, Wiersma M, Kuster DWD, van der Velden J, Brundel BJJM (2019) Untying the knot: protein quality control in inherited cardiomyopathies. Pflugers Arch Eur J Physiol 471:795–806. https://doi.org/10.1007/s00424-018-2194-0
Vincent AE, Grady JP, Rocha MC, Alston CL, Rygiel KA, Barresi R, Taylor RW, Turnbull DM (2016) Mitochondrial dysfunction in myofibrillar myopathy. Neuromuscul Disord 26:691–701. https://doi.org/10.1016/j.nmd.2016.08.004
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy Is Required to Maintain Muscle Mass. Cell Metab 10:507–515. https://doi.org/10.1016/j.cmet.2009.10.008
Levine B, Packer M, Codogno P (2015) Development of autophagy inducers in clinical medicine. J Clin Invest 125:14–24. https://doi.org/10.1172/JCI73938
Glick D, Barth S, Macleod KF (2010) Autophagy: Cellular and molecular mechanisms. J Pathol 221:3–12. https://doi.org/10.1002/path.2697
Parousis A, Carter HN, Tran C, Erlich AT, Mesbah Moosavi ZS, Pauly M, Hood DA (2018) Contractile activity attenuates autophagy suppression and reverses mitochondrial defects in skeletal muscle cells. Autophagy 14:1886–1897. https://doi.org/10.1080/15548627.2018.1491488
Levine B, Kroemer G (2019) Biological Functions of Autophagy Genes: A Disease Perspective. Cell 176:11–42. https://doi.org/10.1016/j.cell.2018.09.048
Rodney GG, Pal R, Abo-Zahrah R (2016) Redox regulation of autophagy in skeletal muscle. Free Radic Biol Med 98:103–112. https://doi.org/10.1016/j.freeradbiomed.2016.05.010
Parzych KR, Klionsky DJ (2014) An overview of autophagy: Morphology, mechanism, and regulation. Antioxidants Redox Signal 20:460–473. https://doi.org/10.1089/ars.2013.5371
Kim YA, Kim YS, Song W (2012) Autophagic response to a single bout of moderate exercise in murine skeletal muscle. J Physiol Biochem 68:229–235. https://doi.org/10.1007/s13105-011-0135-x
Kaludercic N, Maiuri MC, Kaushik S, Fernández ÁF, De Bruijn J, Castoldi F, Chen Y, Ito J, Mukai R, Murakawa T, Nah J, Pietrocola F, Saito T, Sebti S, Semenzato M, Tsansizi L, Sciarretta S, Madrigal-Matute J (2020) Comprehensive autophagy evaluation in cardiac disease models. Cardiovasc Res 116:483–504. https://doi.org/10.1093/cvr/cvz233
Kirkin V (2020) History of the Selective Autophagy Research: How Did It Begin and Where Does It Stand Today? J Mol Biol 432:3–27. https://doi.org/10.1016/j.jmb.2019.05.010
Hale AN, Ledbetter DJ, Gawriluk TR, Rucker EB (2013) Autophagy: Regulation and role in development. Autophagy 9:951–972. https://doi.org/10.4161/auto.24273
Reggiori F, Klionsky DJ (2013) Autophagic processes in yeast: Mechanism, machinery and regulation. Genetics 194:341–361. https://doi.org/10.1534/genetics.112.149013
Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res 24:24–41. https://doi.org/10.1038/cr.2013.168
Zhang J (2015) Teaching the basics of autophagy and mitophagy to redox biologists-Mechanisms and experimental approaches. Redox Biol 4:242–259 https://doi.org/10.1016/j.redox.2015.01.003
Dooley HC, Razi M, Polson HEJ, Girardin SE, Wilson MI, Tooze SA (2014) WIPI2 Links LC3 Conjugation with PI3P, Autophagosome Formation, and Pathogen Clearance by Recruiting Atg12-5-16L1. Mol Cell 55(2):238–252. https://doi.org/10.1016/j.molcel.2014.05.021
Walczak M, Martens S (2013) Dissecting the role of the Atg12-Atg5-Atg16 complex during autophagosome formation. Autophagy 9:424–425. https://doi.org/10.4161/auto.22931
Wei Y, Liu M, Li X, Liu J, Li H (2018) Origin of the Autophagosome Membrane in Mammals. Biomed Res Int 2018. https://doi.org/10.1155/2018/1012789
Tanida I, Ueno T, Kominami E (2008) LC3 and autophagy. Methods Mol Biol 445:77–88. https://doi.org/10.1007/978-1-59745-157-4_4
Kimura S, Noda T, Yoshimori T (2008) Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct Funct 33:109–122. https://doi.org/10.1247/csf.08005
Monastyrska I, Rieter E, Klionsky DJ, Reggiori F (2009) Multiple roles of the cytoskeleton in autophagy. Biol. Rev. 84:431–448. https://doi.org/10.1111/j.1469-185X.2009.00082.x
Tang D, Kang R, Zeh HJ, Lotze MT (2011) High-mobility group box 1, oxidative stress, and disease. Antioxidants Redox Signal 14:1315–1335. https://doi.org/10.1089/ars.2010.3356
Lee J-Y, Koga H, Kawaguchi Y, Tang W, Wong E, Gao Y-S, Pandey UB, Kaushik S, Tresse E, Lu J, Taylor JP, Cuervo AM, Yao T-P (2010) HDAC6 controls autophagosome maturation essential for ubiquitin-selective quality-control autophagy. EMBO J 29:969–980. https://doi.org/10.1038/emboj.2009.405
Mostowy S (2014) Multiple Roles of the Cytoskeleton in Bacterial Autophagy. PLoS Pathog 10:e1004409. https://doi.org/10.1371/journal.ppat.1004409
Shaid S, Brandts CH, Serve H, Dikic I (2013) Ubiquitination and selective autophagy. Cell Death Differ 20:21–30. https://doi.org/10.1038/cdd.2012.72
Mercer EJ, Lin YF, Cohen-Gould L, Evans T (2018) Hspb7 is a cardioprotective chaperone facilitating sarcomeric proteostasis. Dev Biol 435:41–55. https://doi.org/10.1016/j.ydbio.2018.01.005
Matkovich SJ, Van Booven DJ, Hindes A, Kang MY, Druley TE, Vallania FLM, Mitra RD, Reilly MP, Cappola TP, Dorn GW (2010) Cardiac signaling genes exhibit unexpected sequence diversity in sporadic cardiomyopathy, revealing HSPB7 polymorphisms associated with disease. J Clin Invest 120:280–289. https://doi.org/10.1172/JCI39085
Lahvic JL, Ji Y, Marin P, Zuflacht JP, Springel MW, Wosen JE, Davis L, Hutson LD, Amack JD, Marvin MJ (2013) Small heat shock proteins are necessary for heart migration and laterality determination in zebrafish. Dev Biol 384:166–180. https://doi.org/10.1016/j.ydbio.2013.10.009
Hong KW, Lim JE, Kim JW, Tabara Y, Ueshima H, Miki T, Matsuda F, Cho YS., Kim Y, Oh B (2014) Identification of three novel genetic variations associated with electrocardiographic traits (QRS duration and PR interval) in East Asian. Human Mol Genetics 23(24):6659-6667. https://doi.org/10.1093/hmg/ddu374
Ranek MJ, Stachowski MJ, Kirk JA, Willis MS (2018) The role of heat shock proteins and co-chaperones in heart failure. Philos Trans R Soc B Biol Sci 373(1738):20160530. https://doi.org/10.1093/hmg/ddu374
Ulbricht A, Höhfeld J (2013) Tension-induced autophagy: May the chaperone be with you. Autophagy 9:920–922. https://doi.org/10.4161/auto.24213
Schuld J, Orfanos Z, Chevessier F, Eggers B, Heil L, Uszkoreit J, Unger A, Kirfel G, van der Ven PFM, Marcus K, Linke WA, Clemen CS, Schröder R, Fürst DO (2020) Homozygous expression of the myofibrillar myopathy-associated p.W2710X filamin C variant reveals major pathomechanisms of sarcomeric lesion formation. Acta Neuropathol Commun 8(1):154. https://doi.org/10.1186/s40478-020-01001-9
Verdonschot JAJ, Vanhoutte EK, Claes GRF, Helderman-van den Enden ATJM, Hoeijmakers JGJ, Hellebrekers DMEI, de Haan A, Christiaans I, Lekanne Deprez RH, Boen HM, van Craenenbroeck EM, Loeys BL, Hoedemaekers YM, Marcelis C, Kempers M, Brusse E, van Waning JI, Baas AF, Dooijes D, Asselbergs FW, Barge-Schaapveld DQCM, Koopman P, van den Wijngaard A, Heymans SRB, Krapels IPC, Brunner HG (2020) A mutation update for the FLNC gene in myopathies and cardiomyopathies. Hum Mutat 41:1091–1111. https://doi.org/10.1002/humu.24004
Arndt V, Dick N, Tawo R, Dreiseidler M, Wenzel D, Hesse M, Fürst DO, Saftig P, Saint R, Fleischmann BK, Hoch M, Höhfeld J (2010) Chaperone-Assisted Selective Autophagy Is Essential for Muscle Maintenance. Curr Biol 20:143–148. https://doi.org/10.1016/j.cub.2009.11.022
Ulbricht A, Gehlert S, Leciejewski B, Schiffer T, Bloch W, Höhfeld J (2015) Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 11:538–546. https://doi.org/10.1080/15548627.2015.1017186
Bjørkøy G, Lamark T, Brech A, Outzen H, Perander M, Øvervatn A, Stenmark H, Johansen T (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614. https://doi.org/10.1083/jcb.200507002
Bartlett BJ, Isakson P, Lewerenz J, Sanchez H, Kotzebue RW, Cumming R, Harris GL, Nezis IP, Schubert D, Simonsen A, Finley KD (2011) p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy 7:572–583. https://doi.org/10.4161/auto.7.6.14943
Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB (2005) Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell 120:237–248. https://doi.org/10.1016/j.cell.2004.11.046
Mehrbod P, Ande SR, Alizadeh J, Rahimizadeh S, Shariati A, Malek H, Hashemi M, Glover KKM, Sher AA, Coombs KM, Ghavami S (2019) The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 10:376–413. https://doi.org/10.1080/21505594.2019.1605803
Yordy B, Iwasaki A (2011) Autophagy in the control and pathogenesis of viral infection. Curr Opin Virol 1:196–203. https://doi.org/10.1016/j.coviro.2011.05.016
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469:323–335. https://doi.org/10.1038/nature09782
Deretic V, Levine B (2009) Autophagy, Immunity, and Microbial Adaptations. Cell Host Microbe 5:527–549. https://doi.org/10.1016/j.chom.2009.05.016
Wong HH, Sanyal S (2020) Manipulation of autophagy by (+) RNA viruses. Semin. Cell Dev Biol 101:3–11. https://doi.org/10.1016/j.semcdb.2019.07.013
Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T (2004) Autophagy defends cells against invading group A Streptococcus. Science 306:1037–1040. https://doi.org/10.1126/science.1103966
Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C (2005) Escape of intracellular Shigella from autophagy. Science 307:727–731. https://doi.org/10.1126/science.1106036
Gransee HM, Mantilla CB, Sieck GC (2012) Respiratory muscle plasticity. Compr Physiol 2:1441–1462. https://doi.org/10.1002/cphy.c110050
Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524:309–314. https://doi.org/10.1038/nature14893
Moylan JS, Reid MB (2007) Oxidative stress, chronic disease, and muscle wasting. Muscle and Nerve 35:411–429. https://doi.org/10.1002/mus.20743
Di Meo S, Napolitano G, Venditti P (2019) Mediators of physical activity protection against ros-linked skeletal muscle damage. Int J Mol Sci 20(12):3024. https://doi.org/10.3390/ijms20123024
Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, Fisher CC, Zhang M, Saucerman JJ, Goodyear LJ, Kundu M, Yan Z (2017) Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat Commun 8:1–13. https://doi.org/10.1038/s41467-017-00520-9
He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, Levine B (2012) Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481:511–515. https://doi.org/10.1038/nature10758
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy Is Required to Maintain Muscle Mass. Cell Metab 10:507–515. https://doi.org/10.1016/j.cmet.2009.10.008
Maejima Y, Isobe M, Sadoshima J (2016) Regulation of autophagy by Beclin 1 in the heart. J Mol Cell Cardiol 95:19–25. https://doi.org/10.1016/j.yjmcc.2015.10.032
Tannous P, Zhu H, Johnstone JL, Shelton JM, Rajasekaran NS, Benjamin IJ, Nguyen L, Gerard RD, Levine B, Rothermel BA, Hill JA (2008) Autophagy is an adaptive response in desmin-related cardiomyopathy. Proc Natl Acad Sci U S A 105:9745–9750. https://doi.org/10.1073/pnas.0706802105
Jiao J, Demontis F (2017) Skeletal muscle autophagy and its role in sarcopenia and organismal aging. Curr Opin Pharmacol 34:1–6. https://doi.org/10.1016/j.coph.2017.03.009
Demontis F, Perrimon N (2010) FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell 143:813–825. https://doi.org/10.1016/j.cell.2010.10.007
Shirakabe A, Ikeda Y, Sciarretta S, Zablocki DK, Sadoshima J (2016) Aging and Autophagy in the Heart. Circ Res 118:1563–1576. https://doi.org/10.1161/CIRCRESAHA.116.307474
Kakimoto Y, Okada C, Kawabe N, Sasaki A, Tsukamoto H, Nagao R, Osawa M (2019) Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-40250-0
De Meyer GRY, Martinet W (2009) Autophagy in the cardiovascular system. Biochim Biophys Acta - Mol Cell Res 1793:1485–1495. https://doi.org/10.1016/j.bbamcr.2008.12.011
McMillan EM, Quadrilatero J (2014) Autophagy is required and protects against apoptosis during myoblast differentiation. Biochem J 462:267–277. https://doi.org/10.1042/BJ20140312
Ryall JG (2017) Simultaneous measurement of mitochondrial and glycolytic activity in quiescent muscle stem cells. In: Methods in Molecular Biology. Humana Press Inc 1556:245-253. https://doi.org/10.1007/978-1-4939-6771-1_13
Perrotta C, Cattaneo MG, Molteni R, De Palma C (2020) Autophagy in the Regulation of Tissue Differentiation and Homeostasis. Front Cell Dev Biol 8:1563. https://doi.org/10.3389/fcell.2020.602901
Paolini A, Omairi S, Mitchell R, Vaughan D, Matsakas A, Vaiyapuri S, Ricketts T, Rubinsztein DC, Patel K (2018) Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci Rep 8. https://doi.org/10.1038/s41598-018-27429-7
Lee DE, Bareja A, Bartlett DB, White JP (2019) Autophagy as a Therapeutic Target to Enhance Aged Muscle Regeneration. Cells 8(2):183. https://doi.org/10.3390/cells8020183
Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH (2011) Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60:1770–1778. https://doi.org/10.2337/db10-0351
Bibee KP, Cheng YJ, Ching JK, Marsh JN, Li AJ, Keeling RM, Connolly AM, Golumbek PT, Myerson JW, Hu G, Chen J, Shannon WD, Lanza GM, Weihl CC, Wickline SA (2014) Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J 28:2047–2061. https://doi.org/10.1096/fj.13-237388
Cabet E, Batonnet-Pichon S, Delort F, Gausserès B, Vicart P, Lilienbaum A (2015) Antioxidant Treatment and Induction of Autophagy Cooperate to Reduce Desmin Aggregation in a Cellular Model of Desminopathy. PLoS One 10:e0137009. https://doi.org/10.1371/journal.pone.0137009
De Palma C, Morisi F, Cheli S, Pambianco S, Cappello V, Vezzoli M, Rovere-Querini P, Moggio M, Ripolone M, Francolini M, Sandri M, Clementi E (2012) Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 3. https://doi.org/10.1038/cddis.2012.159
Pallafacchina G, Calabria E, Serrano AL, Kalhovde JM, Schiaffino S (2002) A protein kinase B-dependent and rapamycin-sensitive pathway controls skeletal muscle growth but not fiber type specification. Proc Natl Acad Sci U S A 99:9213–9218. https://doi.org/10.1073/pnas.142166599
Barlow AD, Nicholson ML, Herbert TP (2013) Evidence for rapamycin toxicity in pancreatic β-Cells and a review of the underlying molecular mechanisms. Diabetes 62:2674–2682. https://doi.org/10.2337/db13-0106
Pauly M, Daussin F, Burelle Y, Li T, Godin R, Fauconnier J, Koechlin-Ramonatxo C, Hugon G, Lacampagne A, Coisy-Quivy M, Liang F, Hussain S, Matecki S, Petrof BJ (2012) AMPK activation stimulates autophagy and ameliorates muscular dystrophy in the mdx mouse diaphragm. Am J Pathol 181:583–592. https://doi.org/10.1016/j.ajpath.2012.04.004
Kuno A, Hosoda R, Sebori R, Hayashi T, Sakuragi H, Tanabe M, Horio Y (2018) Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin-deficient mdx Mice. Sci Rep 8:1–12. https://doi.org/10.1038/s41598-018-33930-w
Yao Q, Ke ZQ, Guo S, Yang XS, Zhang FX, Liu XF, Chen X, Chen HG, Ke HY, Liu C (2018) Curcumin protects against diabetic cardiomyopathy by promoting autophagy and alleviating apoptosis. J Mol Cell Cardiol 124:26–34. https://doi.org/10.1016/j.yjmcc.2018.10.004
Cescon M, Gattazzo F, Chen P, Bonaldo P (2015) Collagen VI at a glance. J Cell Sci 128:3525–353. https://doi.org/10.1242/jcs.169748
Allamand V, Briñas L, Richard P, Stojkovic T, Quijano-Roy S, Bonne G (2011) ColVI myopathies: where do we stand, where do we go? Skelet Muscle 1:30. https://doi.org/10.1186/2044-5040-1-30
Chrisam M, Pirozzi M, Castagnaro S, Blaauw B, Polishchuck R, Cecconi F, Grumati P, Bonaldo P (2015) Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice. Autophagy 11:2142–2152. https://doi.org/10.1080/15548627.2015.1108508
Fan J, Yang X, Li J, Shu Z, Dai J, Liu X, Li B, Jia S, Kou X, Yang Y, Chen N (2017) Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 8(11):17475–17490. https://doi.org/10.18632/oncotarget.15728
Shen S, Liao Q, Liu J, Pan R, Lee SMY, Lin L (2019) Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J Cachexia Sarcopenia Muscle 10(2):429–444. https://doi.org/10.1002/jcsm.12393
Blokhuis AM, Groen EJN, Koppers M, Van Den Berg LH, Pasterkamp RJ (2013) Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol 125:777–794. https://doi.org/10.1007/s00401-013-1125-6
Gal J, Ström A-L, Kilty R, Zhang F, Zhu H (2007) p62 Accumulates and Enhances Aggregate Formation in Model Systems of Familial Amyotrophic Lateral Sclerosis. J Biol Chem 282:11068–11077. https://doi.org/10.1074/jbc.M608787200
Sasaki S (2011) Autophagy in spinal cord motor neurons in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 70:349–359. https://doi.org/10.1097/NEN.0b013e3182160690
Li Y, Guo Y, Wang X, Yu X, Duan W, Hong K, Wang J, Han H, Li C (2015) Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end-stage amyotrophic lateral sclerosis in a SOD1-G93A mouse model. Neuroscience 298:12–25. https://doi.org/10.1016/j.neuroscience.2015.03.061
Cicardi ME, Cristofani R, Crippa V, Ferrari V, Tedesco B, Casarotto E, Chierichetti M, Galbiati M, Piccolella M, Messi E, Carra S, Pennuto M, Rusmini P, Poletti A (2019) Autophagic and proteasomal mediated removal of mutant androgen receptor in muscle models of spinal and bulbar muscular atrophy. Front Endocrinol (Lausanne) 10:569. https://doi.org/10.3389/fendo.2019.00569
Funding
This work was supported by the Russian Science Foundation, grant No. 20-15-00271.
Author information
Authors and Affiliations
Contributions
Writing a manuscript: K.K.K. and A.I.C.; surveying the relevant literature: K.K.K. and K.S.S.; editing a manuscript: A.A.K.
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare no evident or potential conflict of interest in relation with the publication of this review article.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2021, published in Rossiiskii Fiziologicheskii Zhurnal imeni I.M. Sechenova, 2021, Vol. 107, Nos. 6–7, pp. 810–827https://doi.org/10.31857/S0869813921060042.
Rights and permissions
About this article
Cite this article
Kalugina, K.K., Sukhareva, K.S., Churkinа, A.I. et al. Autophagy as a Pathogenetic Link and a Target for Therapy of Musculoskeletal System Diseases. J Evol Biochem Phys 57, 666–680 (2021). https://doi.org/10.1134/S0022093021030145
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093021030145