Abstract
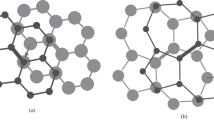
A new two-dimensional hydrocarbon material formed upon complete chemical bonding of hydrogen to the both sides of Stone-Wales graphene, which is a recently predicted new allotrope of graphene, is numerically studied. The band gap Eg = 5.48 eV, the binding energy, and bond lengths, as well as the electron and phonon densities states, are determined. The anisotropy of the Young modulus is revealed. The heating-induced processes of defect formation are studied by the real-time molecular dynamics method. It is shown that the main thermal decomposition channel is the desorption of atomic hydrogen. The second most important decomposition channel is the desorption of molecular hydrogen. For the main decomposition channel, the activation energy Ea = 2.62 eV and the frequency-dependent factor A = 1.1 × 1018 s−1 in the Arrhenius law are determined.
Similar content being viewed by others
References
K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, and A. A. Firsov, Science (Washington, DC, U. S.) 306, 666 (2004).
A. E. Galashev and O. R. Rakhmanova, Phys. Usp. 57, 970 (2014).
K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos, and A. A. Firsov, Nature (London, U.K.) 438, 197 (2005).
G. E. Volovik, JETP Lett. 107, 516 (2018).
X.-L. Sheng, H.-J. Cui, F. Ye, Q.-B. Yan, Q.-R. Zheng, and G. Su, J. Appl. Phys. 112, 074315 (2012).
Y. Liu, G. Wang, Q. Huang, L. Guo, and X. Chen, Phys. Rev. Lett. 108, 225505 (2012).
Z. Wang, X.-F. Zhou, X. Zhang, Q. Zhu, H. Dong, M. Zhao, and A. R. Oganov, Nano Lett. 15, 6182 (2015).
S. Zhang, J. Zhou, Q. Wang, X. Chen, Y. Kawazoe, and P. Jena, Proc. Nat. Acad. Sci. U. S. A. 112, 2372 (2015).
E. A. Belenkov, V. V. Mavrinskii, T. E. Belenkova, and V. M. Chernov, J. Exp. Theor. Phys. 120, 820 (2015).
J. O. Sofo, A. S. Chaudhari, and G. D. Barber, Phys. Rev. B 75, 153401 (2007).
D. C. Elias, R. R. Nair, T. M. G. Mohiuddin, S. V. Morozov, P. Blake, M. P. Halsall, A. C. Ferrari, D. W. Boukhvalov, M. I. Katsnelson, A. K. Geim, and K. S. Novoselov, Science (Washington, DC, U. S.) 323, 610 (2009).
Y. Li, L. Xu, H. Liu, and Y. Li, Chem. Soc. Rev. 43, 2572 (2014).
Y. Gao, T. Cao, F. Cellini, C. Berger, W. A. de Heer, E. Tosatti, E. Riedo, and A. Bongiorno, Nat. Nanotechnol. 13, 133 (2018).
P. V. Bakharev, M. Huang, M. Saxena, S. W. Lee, S. H. Joo, S. O. Park, J. Dong, D. Camacho-Mojica, S. Ji, Y. Kwon, M. Biswal, F. Ding, S. K. Kwak, Z. Lee, and R. S. Ruoff, arXiv: 1901.02131.
K. Kaiser, L. M. Scriven, F. Schulz, P. Gawel, L. Gross, and H. L. Anderson, Science (Washington, DC, U. S.) (2019). https://doi.org/10.1126/science.aay1914
L. A. Chernozatonskii, P. B. Sorokin, A. G. Kvashnin, and D. G. Kvashnin, JETP Lett. 90, 134 (2009).
J. Zhou, Q. Wang, Q. Sun, X. C. Chen, Y. Kawazoe, and P. Jena, Nano Lett. 9, 3867 (2009).
H. Einollahzadeh, S. M. Fazeli, and R. S. Dariani, Sci. Technol. Adv. Mater. 17, 610 (2017).
S. Lebegue, M. Klintenberg, O. Eriksson, and M. I. Katsnelson, Phys. Rev. B 79, 245117 (2009).
A. I. Podlivaev and L. A. Openov, JETP Lett. 106, 110 (2017).
L. A. Openov and A. I. Podlivaev, JETP Lett. 90, 459 (2009).
L. A. Chernozatonskii, P. B. Sorokin, E. E. Belova, J. Bruning, and A. S. Fedorov, JETP Lett. 85, 77 (2007).
H. Yin, X. Shi, C. He, M. Martinez-Canales, J. Li, C. J. Pickard, C. Tang, T. Ouyang, C. Zhang, and J. Zhong, Phys. Rev. B 99, 041405 (2019).
A. J. Stone and D. J. Wales, Chem. Phys. Lett. 128, 501 (1986).
X. Li, Q. Wang, and P. Jena, J. Phys. Chem. Lett. 8, 3234 (2017).
X. Huang, M. Ma, L. Cheng, and L. Liu, Phys. E (Amsterdam, Neth.) 115, 113701 (2020). https://doi.org/10.1016/j.physe.2019.113701
V. I. Artyukhov and L. A. Chernozatonskii, J. Phys. Chem. A 114, 5389 (2010).
L. A. Openov and A. I. Podlivaev, Tech. Phys. Lett. 36, 31 (2010).
L. A. Openov and A. I. Podlivaev, Semiconductors 53, 717 (2019).
L. A. Openov and A. I. Podlivaev, JETP Lett. 109, 710 (2019).
M. M. Maslov, A. I. Podlivaev, and K. P. Katin, Mol. Simul. 42, 305 (2016).
L. A. Openov and A. I. Podlivaev, JETP Lett. 103, 185 (2016).
L. A. Openov and A. I. Podlivaev, JETP Lett. 107, 713 (2018).
M. M. Maslov and K. P. Katin, Chem. Phys. Lett. 644, 280 (2016).
C. D. Reddy, S. Rajendran, and K. M. Liew, Nanotechnology 17, 864 (2006).
K. S. Grishakov, K. P. Katin, V. S. Prudkovskiy, and M. M. Maslov, Appl. Surf. Sci. 463, 1051 (2019).
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 18-02-00278-a) and by the Ministry of Science and Higher Education of the Russian Federation (Program of Excellence for the National Research Nuclear University MEPhI).
Author information
Authors and Affiliations
Corresponding author
Additional information
Russian Text © The Author(s), 2019, published in Pis’ma v Zhurnal Eksperimental’noi i Teoreticheskoi Fiziki, 2019, Vol. 110, No. 10, pp. 692–697.
Rights and permissions
About this article
Cite this article
Podlivaev, A.I. Stone—Wales Graphane: Its Structure, Properties, and Thermal Stability. Jetp Lett. 110, 691–696 (2019). https://doi.org/10.1134/S0021364019220107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0021364019220107