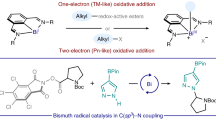
Abstract
The Bu4NI/t-BuOOH oxidative system is widely used in organic synthesis, but mechanistic principles underlying its reactivity are only partially explored. In this work, drawing on the example of the oxidative C–O coupling reaction between compounds with a carbonyl group and (or) a benzyl moiety with N-hydroxyphthalimide, it has been discovered that the coupling with the CH-acidic fragment of the carbonyl group proceeds via ionic mechanism, and the coupling with the benzyl fragment proceeds via radical mechanism. When dimethylacetamide is used as a solvent, the ionic process with the participation of the carbonyl group prevails, while in MeCN the radical process involving the benzyl moiety is realized along with the ionic process. For the oxidative C–O coupling with participation of the benzyl moiety without affecting the α-CH fragment of the carbonyl group, it is advisable to use PhI(OAc)2, Ce(NH4)2(NO2)6, or t‑BuOOt-Bu as oxidants for which only radical pathway is characteristic.



Similar content being viewed by others
REFERENCES
Wu, X.F., Gong, J.L., and Qi, X., Org. Biomol. Chem., 2014, vol. 12, no. 31, pp. 5807–5817. https://doi.org/10.1039/C4OB00276H
Chen, R., Chen, J., Zhang, J., and Wan, X., Chem. Record, 2018, vol. 18, no. 9, pp. 1292–1305. https://doi.org/10.1002/tcr.201700069
Siddaraju, Y. and Prabhu, K.R., Org. Biomol. Chem., 2015, vol. 13, no. 48, pp. 11651–11656. https://doi.org/10.1039/C5OB01929J
Terent’ev, A.O., Zdvizhkov, A.T., Levitsky, D.O., Fleury, F., Pototskiy, R.A., Kulakova, A.N., and Nikishin, G.I., Tetrahedron, 2015, vol. 71, no. 47, pp. 8985–8990. https://doi.org/10.1016/j.tet.2015.09.047
Ma, L., Wang, X., Yu, W., and Han, B., Chem. Commun., 2011, vol. 47, no. 40, pp. 11333–11335. https://doi.org/10.1039/C1CC13568F
Lv, Y., Sun, K., Wang, T., Li, G., Pu, W., Chai, N., Shen, H., and Wu, Y., RSC Adv., 2015, vol. 5, no. 88, pp. 72142–72145. https://doi.org/10.1039/c5ra12691f
Dian, L. and Wang, S., Adv. Synth. Catal., 2015, vol. 357, no. 18, pp. 3836–3842. https://doi.org/10.1002/adsc.201500623
Tan, B., Toda, N., and Barbas, III, C.F., Angew. Chem. Int. Ed., 2012, vol. 51, no. 50, pp. 12538–12541. https://doi.org/10.1002/anie.201205921
Jiang, H., Tang, X., Liu, S., Wang, L., Shen, H., Yang, J., Wang, H., and Gui, Q.W., Org. Biomol. Chem., 2019, vol. 17, no. 48, pp. 10223–10227. https://doi.org/10.1039/c9ob02245g
Minisci, F., Punta, C., Recupero, F., Fontana, F., and Pedulli, G.F., Chem. Commun., 2002, no. 7, pp. 688–689. https://doi.org/10.1039/B110451A
Amorati, R., Lucarini, M., Mugnaini, V., Pedulli, G.F., Minisci, F., Recupero, F., Fontana, F., and Greci, L., Org. Chem., 2003, vol. 68, no. 5, pp. 1747–1754. https://doi.org/10.1021/jo026660z
Minisci, F., Recupero, F., Pedulli, G.F., and Lucarini, M., J. Mol. Catal. A: Chem., 2003, vol. 204, pp. 63–90. https://doi.org/10.1016/S1381-1169(03)00286-3
Bietti, M., Cucinotta, E., DiLabio, G.A., Lanzalunga, O., Lapi, A., Mazzonna, M., Romero-Montalvo, E., and Salamone, M., Org. Chem., 2019, vol. 84, no. 4, pp. 1778–1786. https://doi.org/10.1021/acs.joc.8b02571
Paveliev, S.A., Segida, O.O., Fedorova, U.V., Mulina, O.M., and Terent’ev, A.O., Mendeleev Commun., 2022, vol. 32, no. 2, pp. 167–169. https://doi.org/10.1016/j.mencom.2022.03.004
Krylov, I.B., Lopat’eva, E.R., Budnikov, A.S., Nikishin, G.I., and Terent’ev, A.O., J. Org. Chem., 2019, vol. 85, no. 4, pp. 1935–1947. https://doi.org/10.1021/acs.joc.9b02656
Terent’ev, A.O., Krylov, I.B., Sharipov, M.Y., Kazanskaya, Z.M., and Nikishin, G.I., Tetrahedron, 2012, vol. 68, no. 50, pp. 10263–10271. https://doi.org/10.1016/j.tet.2012.10.018
Qian, P.C., Liu, Y., Song, R.J., Hu, M., Yang, X.H., Xiang, J.N., and Li, J.H., Eur. J. Org. Chem., 2015, vol. 2015, no. 8, pp. 1680–1684. https://doi.org/10.1002/ejoc.201403616
Mazzonna, M., Bietti, M., DiLabio, G.A., Lanzalunga, O., and Salamone, M., Org. Chem., 2014, vol. 79, no. 11, pp. 5209–5218. https://doi.org/10.1021/jo500789v
Kushch, O.V., Hordieieva, I.O., Kompanets, M.O., Zosenko, O.O., Opeida, I.A., and Shendrik, A.N., J. Org. Chem., 2021, vol. 86, no. 5, pp. 3792–3799. https://doi.org/10.1021/acs.joc.0c02595
Yoshino, Y., Hayashi, Y., Iwahama, T., Sakaguchi, S., and Ishii, Y., Org. Chem., 1997, vol. 62, no. 20, pp. 6810–6813. https://doi.org/10.1021/jo9708147
Koshino, N., Cai, Y., and Espenson, J.H., J. Phys. Chem. A, 2003, vol. 107, no. 21, pp. 4262–4267.https://doi.org/10.1021/jp0276193
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Petersson, G.A., Nakatsuji, H., Li, X., Caricato, M., Marenich, A.V., Bloino, J., Janesko, B.G., Gomperts, R., Mennucci, B., Hratchian, H.P., Ortiz, J.V., Izmaylov, A.F., Sonnenberg, J.L., Williams-Young, D., Ding, F., Lipparini, F., Egidi, F., Goings, J., Peng, B., Petrone, A., Henderson, T., Ranasinghe, D., Zakrzewski, V.G., Gao, J., Rega, N., Zheng, G., Liang, W., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Throssell, K., Montgomery, J.A., Jr., Peralta, J.E., Ogliaro, F., Bearpark, M.J., Heyd, J.J., Brothers, E.N., Kudin, K.N., Staroverov, V.N., Keith, T.A., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A.P., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Millam, J.M., Klene, M., Adamo, C., Cammi, R., Ochterski, J.W., Martin, R.L., Morokuma, K., Farkas, O., Foresman, J.B., and Fox, D.J., Gaussian 16, Revision A.03, Gaussian Inc., Wallingford CT, 2016.
Montgomery, J.A., Jr., Frisch, M.J., Ochterski, J.W., and Petersson, G.A., J. Chem. Phys., 2000, vol. 112, no. 15, pp. 6532–6542. https://doi.org/10.1063/1.481224
Montgomery, J.A., Jr., Frisch, M.J., Ochterski, J.W., and Petersson, G.A., J. Chem. Phys., 1999, vol. 110, no. 6, pp. 2822–2827. https://doi.org/10.1063/1.477924
Chai, J.-D. and Head-Gordon, M., Phys. Chem. Chem. Phys., 2008, vol. 10, no. 44, pp. 6615–6620. https://doi.org/10.1039/B810189B
Marenich, A.V., Cramer, C.J., and Truhlar, D.G., J. Phys. Chem. B, 2009, vol. 113, no. 18, pp. 6378–6396. https://doi.org/10.1021/jp810292n
Funding
This work was supported by the Russian Science Foundation (Grant no. 21‑13‑00205).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Additional information
This work was submitted to the thematic issue “Free radicals in basic and applied chemistry.”
Rights and permissions
About this article
Cite this article
Lopat’eva, E.R., Krylov, I.B., Kuzmin, I.V. et al. Oxidative C–O Coupling: Radical and Ionic Pathways of Reaction in Bu4NI/t-BuOOH System. Dokl Chem 504, 67–73 (2022). https://doi.org/10.1134/S0012500822600092
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012500822600092