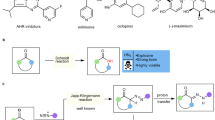
Abstract
A simple method has been developed for homocoupling of terminal alkynes bearing different functional groups by reaction with CuI/tribromoisocyanuric acid/piperidine in acetonitrile at room temperature. A telescoped approach based on Hunsdiecker/Cadiot–Chodkiewicz reactions for C(sp)-C(sp) cross-coupling was also presented.
Graphic abstract


Similar content being viewed by others
References
Shun ALKS, Tykwinski RR (2006) Angew Chem Int Ed 45:1034
Zhou Z-F, Menna M, Cai Y-S, Guo Y-W (2015) Chem Rev 115:1543
Mao J, Li S, Zhong J, Wang B, Jin J, Gao Z, Yang H, Bian Q (2016) Tetrahedron Asymmetry 27:69
Shi W, Lei A (2014) Tetrahedron Lett 55:2763
Andrade CB, Carvalho DB, Trefzger OS, Kassab NM, Guerrero PG, Barbosa SL, Shiguemoto CYK, Baroni ACM (2019) Eur J Org Chem 696
Ötvös SB, Georgiádes A, Ozsvár D, Fülöp F (2019) RSC Adv 9:8197
Glaser C (1869) Ber Dtsch Chem Ges 2:422
Eglinton G, Galbraith AR (1956) Chem Ind 737
Hay AS (1962) J Org Chem 27:3320
Chodkiewicz W, Cadiot C (1955) C R Hebd Seances Acad Sci 241:1055
For a review on copper acetylides in organic synthesis, see: Adeleke AF, Brown APN, Cheng LJ, Mosleh KAM, Cordier CJ (2017) Synthesis 49:790
For a review on the Glaser coupling reaction, see: Sindhu KS, Anilkumar G (2014) RSC Adv 4:27867
Bettanin L, Botteselle GV, Godoi M, Braga AL (2014) Green Chem Lett Rev 7:105
Kusuda A, Xu X-H, Wang X, Tokunaga E, Shibate N (2011) Green Chem 13:843
Li D, Yin K, Li J, Jia X (2008) Tetrahedron Lett 49:5918
Li L, Wang J, Zhang G, Liu Q (2009) Tetrahedron Lett 50:4033
Fan X, Li N, Shen T, Cui X-M, Lv H, Zhu H-B, Guan Y-H (2014) Tetrahedron 70:256
Li JX, Liang HR, Wang ZY, Fu J-H (2011) Monatsh Chem 142:507
Kaldhi D, Vodnala N, Arup RG, Kabi K, Nayak S, Malakar CC (2020) Tetrahedron Lett 61:151775 (and references cited therein)
For a review on the chemistry of tribromoisocyanuric acid, see: de Almeida LS, Esteves PM, de Mattos MCS (2014) Curr Green Chem 1:94
Tozetti SDF, de Almeida LS, Esteves PM, de Mattos MCS (2007) J Braz Chem Soc 18:675
Sindra HC, dos Santos CVP, de Mattos MCS (2020) Lett Org Chem 17:586
de Andrade VSC, de Mattos MCS (2020) Tetrahedron Lett 61:152164
Crespo LTC, Senra MR, Esteves PM, de Mattos MCS (2019) Lett Org Chem 16:627
Sanabria CM, Costa BBS, Viana GM, de Aguiar LCS, de Mattos MCS (2018) Synthesis 50:1359
de Almeida LS, Esteves PM, de Mattos MCS (2006) Synthesis 221
Waminathan S, Narayanan KV (1971) Chem Rev 71:429
Yin W, He C, Chen M, Zhang H, Lei A (2008) Org Lett 11:709
Balaraman K, Kesavan V (2010) Synthesis 3461
Su L, Dong J, Liu L, Sun M, Qiu R, Zhou Y, Yin S-F (2016) J Am Chem Soc 138:12348
For a recent review on the Cadiot-Chodkiewicz reaction, see: Radhika S, Harry NA, Neetha M, Anilkumar G (2019) Org Biomol Chem 17:9081
Hayashi Y (2016) Chem Sci 7:866
Sodré LR, Esteves PM, de Mattos MCS (2013) J Braz Chem Soc 24:212
Rossi R, Carpita A, Bigelli C (1985) Tetrahedron Lett 26:523
Bohlmann F, Schönowsky H, Inhoffen E, Grau G (1964) Chem Ber 97:794
Jover J, Spuhler P, Zhao L, McArdle C, Maseras F (2014) Catal Sci Technol 4:4200
de Almeida LS, Esteves PM, de Mattos MCS (2006) Synlett 1515
Susanto W, Chu C-Y, Ang WJ, Chou TC, Lo L-C, Lam Y (2012) J Org Chem 77:2729
Tang S, Li L, Ren X, Li J, Yang G, Li H, Yuan B (2019) Green Chem 21:2899
Mo G, Tian Z, Li J, Wen G, Yang X (2015) Appl Organomet Chem 29:231
Kusuda A, Xu X-H, Wang X, Tokunaga E, Shibata N (2011) Green Chem 13:843
Li J-H, Liang Y, Zhang X-D (2005) Tetrahedron 61:1903
Adimurthy S, Malakar CC, Beifuss U (2009) J Org Chem 74:5648
Xu R, Gramlich V, Frauenrath H (2006) J Am Chem Soc 128:5541
Li M, Li Y, Zhao B, Liang F, Jin L-Y (2014) RSC Adv 4:30046
Acknowledgements
We thank CNPq and CAPES for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Andrade, V.S.C., de Mattos, M.C.S. Tribromoisocyanuric acid as a useful oxidant for the synthesis of 1,3-diynes via Glaser coupling. Monatsh Chem 151, 1403–1408 (2020). https://doi.org/10.1007/s00706-020-02673-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02673-8