Abstract
The electron paramagnetic resonance (EPR) method is widely used in the biophysics of photosynthesis, because it serves as an effective tool for exploring the processes of electron and proton transport in various photosynthetic systems. This study on the regulation of electron transport in chloroplasts was performed with the direct participation of the authors using the EPR method. The possibilities of the EPR method to study the kinetics of electron transport in chloroplasts of higher plants in situ (leaves of higher plants) at room temperature were shown, and the EPR spectra of chloroplasts at cryogenic temperatures were considered. The latter is of particular importance for substantiating the “kinetic” method of pH measurement inside thylakoids, which was used.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
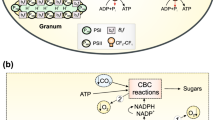
The processes of oxygenic photosynthesis in higher plants occur in chloroplasts, specialized energy-transforming organelles of plant cells [1]. A schematic representation of the chloroplast is shown in Fig. 1a. Under the double envelope of the chloroplast, consisting of the outer and inner membranes, there are extended membrane structures that form closed vesicles shaped like disks and called thylakoids. Thylakoid membranes contain photosynthetic pigment-protein complexes (Fig. 1b). In higher plants, thylakoids are grouped into grana, stacks of flattened and closely pressed together thylakoids. The intergrana (stromal) thylakoids are the continuation of the individual thylakoids of the grana that protrude. The stroma (the space between the chloroplast envelope and the thylakoids) contains RNA, DNA, ribosomes, starch grains, and numerous enzymes that ensure the absorption of CO2 by plants. Thylakoid membranes contain pigment-protein complexes involved in photosynthetic electron transport, as well as ATP-synthase complexes that catalyze the formation of ATP from ADP and inorganic phosphate (Pi).
Biophysical research methods have played a crucial role in elucidating the mechanisms of electron transport in plants. The electron-transport chain carriers of chloroplasts were identified and characterized using high-time-resolution absorption spectroscopy; fluorescence analysis of the pigments of the photosynthetic apparatus underlies diagnosis of the functional state of chloroplasts in vivo. A special role in the study of oxygenic photosynthesis is played by the electron paramagnetic resonance (EPR) method, with which electron carriers, that are practically invisible to traditional optical methods since the relatively weak absorption spectra of these molecules are masked by the intense absorption spectra of the photosynthetic pigments and cytochromes, were identified in chloroplasts. These carriers include electron transport cofactors containing nonheme iron, proteins included in the pigment-protein complex of photosystem I (PS I) [2], the copper-containing protein plastocyanin [3], and the iron-sulfur cluster (Fe2S2) of the Rieske protein [4] that is included in the cytochrome b6 f-complex of chloroplasts [5–8].
L.A. Blumenfeld is a leader in the study of biological systems using the EPR method. He was one of the first who started this research in the late 1950s (see the essay by S.E. Shnol in the book [9]). A significant part of L.A. Blumenfeld’s research was devoted to the study of photosynthetic systems using EPR [10–20]. These studies have been carried out for many years at the Department of Biophysics of the Faculty of Physics of Lomonosov Moscow State University and at the Institute of Chemical Physics of the Academy of Sciences. It is not possible to cover the wide variety of studies in the field of photosynthesis biophysics carried out under the leadership of L.A. Blumenfeld in a short article. We will note only some of the most important scientific achievements in this field. The kinetics of photoinduced P700 transformations in chloroplasts of higher plants in situ (leaves) was studied in detail using the EPR method, and two sites of regulation of photosynthetic electron transfer were identified, the acceptor site of PS I and the stage of oxidation of plastoquinol (PQH2) by the cytochrome b6 f complex [21–24]. In the work of Blumenfeld with the staff of the Institute of Chemical Physics [20], the functioning of a two-electron “gate” associated with the two-electron reduction of plastoquinone (PQ) at the acceptor site of photosystem II (PS II) to its fully reduced form, plastoquinol (PQH2) was shown. Plastoquinol is oxidized by a cytochrome b6 f-complex containing an iron-sulfur Fe2S2 cluster of the Rieske protein. By analyzing the relaxation characteristics of Fe2S2 centers in the electron transport chains of mitochondria and chloroplasts, Blumenfeld suggested that relatively slow conformational rearrangements of the protein globule occur in the Rieske proteins of the bc1 and b6 f cytochrome complexes. According to the relaxation concept of enzymatic catalysis put forward by Blumenfeld [11–14], these rearrangements are initiated by rapid electron transport reactions in the electron transport chains of mitochondria and chloroplasts. Subsequently, the assumption of conformational changes in the Rieske protein found convincing experimental evidence [25] (see also review articles [5–8]).
L.A. Blumenfeld was very interested in biophysical studies of photosynthesis, which were conducted at the Department of Biophysics of the Faculty of Physics of Lomonosov Moscow State University. It is not possible to write about about all the directions of these studies in a single short article. We will focus on the works devoted to the study of the regulation of electron transport in chloroplasts using the EPR method, which were carried out under the guidance and with the direct participation of the authors of this article. We will illustrate the possibilities of the EPR method in the study of the kinetics of electron transport in chloroplasts of higher plants in situ (leaves of higher plants) at room temperatures, and consider the EPR spectra of chloroplasts at cryogenic temperatures. The latter is of particular importance for substantiating the “kinetic” method of pH measurement inside thylakoids, which we used.
MATERIALS AND METHODS
The objects of the study were the leaves of a Chinese rose houseplant (Hibiscus rosa-sinensis), bean leaves (Vicia faba) and chloroplasts isolated from them. The method of isolation of class B chloroplasts from the seedlings of the “Russian Black” bean variety was described in [18, 26]. Chloroplasts were suspended in an incubation medium containing 10 mM tricin buffer (pH 7.5). A 10 μM solution of methylviogen was used as an artificial electron acceptor in PS I. EPR spectra were recorded at room temperature (22–24 °C) at a microwave power of 10 mW and an HF modulation amplitude equal to 0.4 mT. To record the kinetics of redox transformations of the photoreaction center of photosystem I (P700), the magnetic field was fixed at the low-field extremum of the EPR signal from the oxidized centers of \({\text{P}}_{{700}}^{ + }\). The samples were illuminated with white light from an incandescent lamp (300 W), exciting both photosystems, or with a far-red light (λmax = 707 nm, 8 W), obtained with an IF707 interference filter (Karl Zeiss Jena, Germany), which excited mainly PS I.
Measurements of the EPR spectra of the studied objects were carried out using E-4 and E-9E EPR spectrometers (Varian, United States), equipped with prefixes for varying the temperature of samples in a wide range. Measurements of EPR spectra at cryogenic temperatures were carried out using the ESR-9 installation (Oxford Instruments, United Kingdom). Preparations of isolated chloroplasts were placed in quartz ampoules and adapted to the dark or illumination with white light for 1 min. The ampoules with the samples were quickly frozen by cooling in liquid butane to 77 K and placed in the resonator of the E-109E spectrometer. The temperature of the measured samples was varied in the range of 6–30 K, microwave power was in the range of 0.01–10 mW. The temperature of the samples in the resonator of the spectrometer was measured using a calibrated thermocouple. Particular attention was paid to the careful selection of quartz ampoules that did not give parasitic EPR signals at cryogenic temperatures.
RESULTS AND DISCUSSION
Kinetics of Photoinduced Redox Transformations of P700 in Chloroplasts
Figure 2 shows the EPR spectra of bean chloroplasts and the kinetics of photoinduced changes in the magnitude of the EPR signal from oxidized \({\text{P}}_{{700}}^{ + }\) centers in the leaves and isolated bean chloroplasts. It is seen from Fig. 2a that the illumination of chloroplasts with a far-red light (λmax = 707 nm) caused oxidation of P700. This was clearly evidenced by the parameters of the EPR signal induced by the far-red light (the value of the g-factor was 2.0025 and the half-width of the signal ΔHpp = 0.9 mT) [27]. In response to flashes of white light of various durations delivered under far red-light illumination, \({\text{P}}_{{700}}^{ + }\) was first reduced followed by the oxidation of P700 due to the action of light with λmax = 707 nm. Nonmonotonic photoinduced changes in the EPR signal from \({\text{P}}_{{700}}^{ + }\) occurred after switching on the continuous white light. Multiphase kinetics that included several stages of signal magnitude changes (curve A–B—K–L–C was observed in bean leaves) (Fig. 2b). A simpler kinetic curve (the so-called A–B–C “overlap”) was observed in the case of isolated Class B chloroplasts (Fig. 2c).
EPR spectra of bean chloroplasts. The first derivative of the absorption spectrum (a), and the kinetics of photoinduced changes in the magnitude of the EPR signal from \({\text{P}}_{{700}}^{ + }\) in leaves (b) and isolated bean chloroplasts (c). Zigzag arrows show the moments of white light flashes with a duration of t1/2 = 7 μs (1) and t1/2 = 750 μs (2).
We have studied the dependence of the kinetics of redox transformations of P700 on the prehistory of the illumination of samples (the duration of adaptation to darkness or light of a certain spectral composition) and on the action of inhibitors or mediators of electron transport, as well as uncouplers affecting the transmembrane difference of electrochemical potential in chloroplasts (see more in [22–24, 28–32]). It was found out which factors of regulation of electron transport determine the nonmonotonic kinetics of changes in the EPR signal from \({\text{P}}_{{700}}^{ + }\) in leaf chloroplasts adapted to darkness. The main contribution to the observed phenomena was made by 1) the photoinduced increase in the activity of the enzymes of the Calvin-Benson cycle, which caused an acceleration of the outflow of electrons from PS I; and 2) a decrease in the rate of electron influx to \({\text{P}}_{{700}}^{ + }\) [18, 23, 24, 32]. The reduction of the electron flow from PS II to PS I can occur for various reasons, including a decrease in the photochemical activity of PS II due to increased non-photochemical quenching of chlorophyll excitation in the light-harvesting antenna of PS II and a slowdown in the rate of oxidation of plastoquinol (PQH2) by the cytochrome b6 f complex as a result of photoinduced acidification of the intra-thylakoid space (pHin) [33–37].
The latter was of particular interest, since the “kinetic” method of noninvasive pHin measurement in chloroplasts in situ and in vitro is based on this phenomenon. The method is based on measurements of the rate of electron transport to \({\text{P}}_{{700}}^{ + }\), depending on pHin [18, 29, 34]. The “kinetic” method of pHin measurement was used in our work to measure the trans-thylakoid pH difference (ΔpH) in various metabolic states of chloroplasts (see the review [37] for more detail). One of the conditions for the adequate use of this method is that the state of electron carriers in both coupled and uncoupled chloroplasts used to construct the calibration dependence of the electron transportin rate between PS II and PS I on pHin should be the same. To substantiate this method, we carried out the measurements of the low-temperature EPR spectra of chloroplasts described below.
Redox State of Electron Transport Chain Carriers (According to Low-Temperature EPR Spectroscopy)
Figure 3 shows the EPR spectra of bean chloroplasts reduced by dithionite and frozen during their illumination with white light. Intense lines belonging to the reduced acceptors of PS I, iron-sulfur centers FA and FB, were visible in the EPR spectrum. Comparison of the g-factors of these signals with the literature data indicated that both interacting and non-interacting with each other FA and FB centers (signals with g = 1.94 and g = 1.92) could contribute to the observed spectrum (for identification of EPR lines in chloroplasts at cryogenic temperatures, see review papers [38–40]). As expected, there was no signal in the EPR spectrum that could belong to the fraction of water-soluble ferredoxin washed out during the isolation of Class B chloroplasts. The most intense signal line with gy = 1.89–1.90 was close to the band with gx = 1.89 belonging to the acceptor FB and/or FA (provided that it interacted with the reduced acceptor FB).
To unambiguously attribute the signal with gy = 1.90 to the reduced Fe2S2 cluster of the Rieske protein, we needed to prove that it differed in its characteristics from the component of the signal with gx = 1.89 belonging to the reduced FB center. We were able to verify this by measuring the dependences of the line intensities with different values of the g-tensor depending on the microwave power and temperature of the sample (data not presented).
The upper part of Fig. 4 shows the EPR spectra in chloroplasts frozen in the dark after preliminary illumination of chloroplasts with white light in the presence of gramicidin D. The signals belonging to the oxidized plastocyanin (g = 2.05), the reduced Fe-S center of FA (g = 1.94) and the reduced Rieske center (g = 1.90) were clearly visible. That the signal with g = 1.90 belonged to the Rieske center was proven by the fact that this signal was observed in chloroplasts incubated in the dark in the presence of 10 mM ascorbate. In this case, the EPR signal lines with g = 1.94 and g = 1.92 related to the reduced centers FA and FB were missing. At the same time, along with the line at g = 1.90, a component with g = 2.02 was observed, belonging to the low-field component of the EPR signal from the reduced iron-sulfur cluster of the Rieske protein.
Figure 5 shows fragments of EPR spectra of chloroplast samples for signal components with g = 2.05 (oxidized plastocyanin) and with g = 1.90 (the reduced Fe2S2 center of the Rieske protein). The samples were obtained by rapidly freezing chloroplasts in different metabolic states in the light or in the dark (30 s after the light was turned off). It can be seen from these data that during the illumination of chloroplasts with continuous white light, most of the plasticyanin and Fe2S2 molecules in the Rieske center were in an oxidized state. After the light was turned off, these carriers were reduced due to the plastoquinone pool, which was maintained, at least partially, in a reduced state. We can assess the state of the plastoquinone pool under illumination conditions by the kinetics of redox transformations of P700, shown in Fig. 2c. The rapid reduction of \({\text{P}}_{{700}}^{ + }\) immediately after switching off the white light indicated that there were reduced plastoquinol molecules in the electron transport chain between PS II and PS I.
EPR spectra of bean chloroplasts measured at 20 K. The samples were obtained by rapidly freezing suspensions of chloroplasts that were in different metabolic states under illumination and in the dark (after the light was turned off). Control, chloroplasts without additives; chloroplasts under conditions of ATP synthesis (in the presence of 4 mM Mg-ADP); uncoupled chloroplasts (in the presence of 10 μM of gramicidin D).
The data shown in Fig. 5 indicate that in all three metabolic states, in the control (without additives), under conditions of photophosphorylation (in the presence of 4 mM Mg-ADP) and in uncoupled chloroplasts (with the addition of 10 μM of gramicidin D), the state of the donor section of the electron transport chain between the cytochrome b6 f complex and the P700 was almost the same. Based on this, we can assert that one of the basic requirements necessary for the correct measurement of intra-thylakoid pH (pHin) by the “kinetic” method was fulfilled in our experiments. The difference in the reduction rates of \({\text{P}}_{{700}}^{ + }\), which we observed in different metabolic states of chloroplasts [18, 29], was due to different values of the intra-thylakoid pHin, and not to different states of the electron transport chain in the region between the cytochrome b6 f-complex and P700. It should be noted that the “kinetic” method of pHin measurement we have developed has one indisputable advantage, namely, it can be used to carry out non-invasive (in situ) pHin measurements in chloroplasts located in leaves.
CONCLUSIONS
It should be noted that the EPR method has found wide application in the biophysics of photosynthesis, since it serves as an effective tool for studying the processes of electron and proton transport in various photosynthetic systems. Above, we have considered only some directions in the research of bioenergetic processes in photosynthetic systems using the EPR method to record electron transport processes in chloroplasts and the leaves of higher plants. Other possibilities for the EPR method in the study of photosynthesis are related to using stable radicals (spin probes). With the help of stable radicals serving as paramagnetic probes sensitive to their local environment, it is possible to monitor structural rearrangements in pigment-protein complexes, as well as to measure the trans-thylakoid difference in the electrochemical potentials of hydrogen ions [37, 41].
Change history
19 December 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S0006350922330016
REFERENCES
G. Edwards and D. Walker, Photosynthesis of C3 and C4 Plants: Mechanisms and Regulation (Mir, Moscow, 1986).
R. Malkin and A. J. Bearden, Proc. Natl. Acad. Sci. U. S. A. 68, 16 (1971).
R. Malkin and A. J. Bearden, Biochim. Biophys. Acta 292, 169 (1973).
R. Malkin and P. J. Aparicio, Biochim. Biophys. Res. Commun. 63, 1157 (1975).
W.A. Cramer and S. S. Hasan, Adv. Photosynth. Respir. 41, pp.177–207 (2016).
A. N. Tikhonov, in Membrane Protein Complexes: Structure and Function, Sub-Cellular Biochemistry, Ed. By J. R. Harris and E. J. Boekema (Springer-Verlag, Singapore, 2018), Vol. 87, pp. 287–328.
L. A. Malone, M. S. Proctor, A. Hitchcock, et al., Biochim. Biophys. Acta, Bioenerg. 1862, 148380 (2021).
M. Sarewicz, S. Pintscher, R. Pietras, et al., Chem. Rev. 121, 2020 (2021).
S. E. Shnol’, Heroes, Villains, Conformists of National Science, 6th ed., (URSS, Moscow, 2022).
L. A. Blumenfeld, Izv. Akad. Nauk SSSR, Ser. Biol. No. 3, 285 (1957).
L. A. Blumenfeld, Problems of Biological Physics (Nauka, Moscow, 1974).
L. A. Blumenfeld, Physics of Bioenergetic Processes (Springer-Verlag, Heidelberg, 1983).
L. A. Blumenfeld and A. N. Tikhonov, Biophysical Thermodynamics of Intracellular Processes. Molecular Machines of the Living Cell (Springer-Verlag, New-York, 1994).
L. A. Blumenfeld, R. M. Davydov, and A. N. Tikhonov, J. Mol. Liq. 42, 231 (1989).
E. K. Ruuge, A. N. Tikhonov, and L. A. Blumenfeld, Biofizika 19, 938 (1974).
E. K. Ruuge, A. N. Tikhonov, and L. A. Blumenfeld, Biofizika 19, 1033 (1974).
A. N. Tikhonov, E. K. Ruuge, V. K. Subchinski, et al., Fiziol. Rast. 22, 5 (1975).
A. N. Tikhonov, G. B. Khomutov, E. K. Ruuge, et al., Biochim. Biophys. Acta 637, 321 (1981).
A. N. Tikhonov, A. A. Timoshin, L. A. Blumenfeld, Mol. Biol. 17, 1236 (1983).
L. A. Blumenfeld, M. G. Goldfield, A. I. Tzapin, et al., Photosynthetica 8, 168 (1974).
A. N. Tikhonov and E. K. Ruuge, Biofizika 20, 1049 (1975).
E. K. Ruuge and A. N. Tikhonov, Biofizika 22, 268 (1977).
S. B. Ryzhikov and A. N. Tikhonov, Biofizika 33, 642 (1988).
A. N. Tikhonov, Photosynth. Res. 125, 65 (2015).
Z. Zhang, L. Huang, V. M. Schulmeister, et al., Nature 392, 677 (1998).
S. Izawa, R. Kraayehof, E. K. Ruuge, et al., Biochim. Biophys. Acta 314 (3), 328 (1973).
A. N. Webber and W. Lubitz, Biochim. Biophys. Acta 1507, 61 (2001).
A. N. Tikhonov and E. K. Ruuge, Biofizika 22, 839 (1978).
A. N. Tikhonov, G. B. Khomutov, and E. K. Ruuge, Photobiochem. Photobiophys. 8, 261 (1984).
A. N. Tikhonov., G. B. Khomutov, and E. K. Ruuge, Mol. Biol. 13, 1085 (1979).
G. B. Khomutov, A. N. Tikhonov, and E. K. Ruuge, Mol. Biol. 15, 182 (1981).
B. V. Trubitsin, A. V. Vershubskii, V. I. Priklonskii, et al., J. Photochem. Photobiol., B 152, 400 (2015).
B. Rumberg and U. Siggel, Naturwissenschaften 56, 130 (1969).
A. N. Tikhonov, Photosynth. Res. 116, 511 (2013).
D. M. Kramer, C. A. Sacksteder, and J. A. Cruz, Photosynth. Res. 60, 151 (1999).
A. N. Tikhonov, R. V. Agafonov, L. A. Grigor’ev, et al., Biochim. Biophys. Acta 1777, 285 (2008).
A. N. Tikhonov, Cell Biochem. Biophys. 75, 421 (2017).
K. Brettel, Biochim. Biophys. Acta 1318, 322 (1997).
J. H. Golbeck, Photosynth. Res. 61, 107 (1999).
I. R. Vassiliev, M. L. Antonkine, and J. H. Golbeck, Biochim. Biophys. Acta 1507, 139 (2001).
A. N. Tikhonov and W. K. Subczynski, in Biomedical EPR – Part A: Free Radicals, Metals, Medicine, and Physiology, Ed. by S. S. Eaton, G. R. Eaton, and L. J. Berliner (Kluwer Academic/Plenum Publishers, Boston, 2005), Vol. 23, pp. 147–194.
ACKNOWLEDGMENTS
This paper is dedicated to the 100th anniversary of the birth of our teacher, Lev Alexandrovich Blumenfeld, one of the pioneers of the use of EPR in the study of biological systems. We are grateful to our colleagues, G.B. Khomutov, A.A. Timoshin and S.B. Ryzhikov, with whom part of our early work on the study of the mechanisms of regulation of electron transport in chloroplasts of higher plants, which we refer to in this paper, was carried out. We are also grateful to Yu.A. Koksharov and B.V. Trubitsin for the computerization of the E4 and E-109E EPR spectrometers used in our work. We also give special thanks to B.V. Trubitsin for his help in digitizing EPR spectra.
Funding
The work was carried out with the financial support of the Russian Science Foundation, project no. 21-74-20047.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving humans or animals as research objects.
Additional information
Translated by E. Puchkov
Abbreviations: EPR, electronic paramagnetic resonance; PS I, photosystem I; PS II, photosystem II.
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruuge, E.K., Tikhonov, A.N. Electron Paramagnetic Resonance: Study of the Regulatory Mechanisms of Light Phases of Photosynthesis in Plants. BIOPHYSICS 67, 406–412 (2022). https://doi.org/10.1134/S0006350922030186
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350922030186