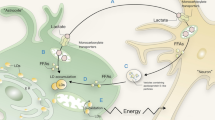
Abstract
Lipids comprise an extremely heterogeneous group of compounds that perform a wide variety of biological functions. Traditional view of lipids as important structural components of the cell and compounds playing a trophic role is currently being supplemented by information on the possible participation of lipids in signaling, not only intracellular, but also intercellular. The review article discusses current data on the role of lipids and their metabolites formed in glial cells (astrocytes, oligodendrocytes, microglia) in communication of these cells with neurons. In addition to metabolic transformations of lipids in each type of glial cells, special attention is paid to the lipid signal molecules (phosphatidic acid, arachidonic acid and its metabolites, cholesterol, etc.) and the possibility of their participation in realization of synaptic plasticity, as well as in other possible mechanisms associated with neuroplasticity. All these new data can significantly expand our knowledge about the regulatory functions of lipids in neuroglial relationships.



Similar content being viewed by others
Abbreviations
- Apo:
-
apolipoprotein
- AA:
-
arachidonic acid
- CNS:
-
central nervous system
- DGK:
-
diglycerol kinase
- LPs:
-
lipoproteins
- OLs:
-
oligodendrocytes
- PA:
-
phosphatidic acid
References
Barres, B. A. (2008) The mystery and magic of glia: a perspective on their roles in health and disease, Neuron, 60, 430-440, https://doi.org/10.1016/j.neuron.2008.10.013.
Jakel, S., and Dimou, L. (2017) Glial cells and their function in the adult brain: a journey through the history of their ablation, Front. Cell Neurosci., 11, 24, https://doi.org/10.3389/fncel.2017.00024.
Hatton, G. I. (2002) Glial-neuronal interactions in the mammalian brain, Adv. Physiol. Educ., 26, 225-237, https://doi.org/10.1152/advan.00038.2002.
Galkina, O. V., Vetrovoy, O. V., and Eschenko, N. D. (2021) The role of lipids in implementing specific functions in the central nervous system, Russ. J. Bioorg. Chem., 47, 1004-1013.
Sofroniew, M. V., and Vinters, H. V. (2010) Astrocytes: biology and pathology, Acta Neuropathol., 119, 7-35, https://doi.org/10.1007/s00401-009-0619-8.
Von Bartheld, C. S., Bahney, J., and Herculano-Houzel, S. (2016) The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting, J. Comp. Neurol., 524, 3865-3895, https://doi.org/10.1002/cne.24040.
Abbott, N. J., Ronnback, L., and Hansson, E. (2006) Astrocyte-endothelial interactions at the blood-brain barrier, Nat. Rev. Neurosci., 7, 41-53, https://doi.org/10.1038/nrn1824.
Rothstein, J. D., Dykes-Hoberg, M., Pardo, C. A., Bristol, L. A., Jin, L., Kuncl, R. W., Kanai, Y., Hediger, M. A., Wang, Y., Schielke, J. P., and Welty, D. F. (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate, Neuron, 16, 675-686, https://doi.org/10.1016/s0896-6273(00)80086-0.
Simard, M., and Nedergaard, M. (2004) The neurobiology of glia in the context of water and ion homeostasis, Neuroscience, 129, 877-896, https://doi.org/10.1016/j.neuroscience.2004.09.053.
Butt, A. M., and Kalsi, A. (2006) Inwardly rectifying potassium channels (Kir) in central nervous system glia: a special role for Kir4.1 in glial functions, J. Cell Mol. Med., 10, 33-44, https://doi.org/10.1111/j.1582-4934.2006.tb00289.x.
Hewett, J. A. (2009) Determinants of regional and local diversity within the astroglial lineage of the normal central nervous system, J. Neurochem., 110, 1717-1736, https://doi.org/10.1111/j.1471-4159.2009.06288.x.
Allen, N. J., and Eroglu, C. (2017) Cell biology of astrocyte-synapse interactions, Neuron, 96, 697-708, https://doi.org/10.1016/j.neuron.2017.09.056.
Pannasch, U., Vargová, L., Reingruber, J., Ezan, P., Holcman, D., Giaume, C., Syková, E., and Rouach, N. (2011) Astroglial networks scale synaptic activity and plasticity, Proc. Natl. Acad. Sci. USA, 108, 8467-8472, https://doi.org/10.1073/pnas.1016650108.
Varcianna, A., Myszczynska, M. A., Castelli, L. M., O’Neill, B., Kim, Y., Talbot, J., Nyberg, S., Nyamali, I., Heath, P. R., Stopford, M. J., Hautbergue, G. M., and Ferraiuolo, L. (2019) Micro-RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS, EBioMedicine, 40, 626-635, https://doi.org/10.1016/j.ebiom.2018.11.067.
Ullian, E. M., Sapperstein, S. K., Christopherson, K. S., and Barres, B. A. (2001) Control of synapse number by glia, Science, 291, 657-661, https://doi.org/10.1126/science.291.5504.657.
Hu, R., Cai, W. Q., Wu, X. G., and Yang, Z. (2007) Astrocyte-derived estrogen enhances synapse formation and synaptic transmission between cultured neonatal rat cortical neurons, Neuroscience, 144, 1229-1240, https://doi.org/10.1016/j.neuroscience.2006.09.056.
Araque, A. (1999) Tripartite synapses: glia, the unacknowledged partner, Trends Neurosci., 22, 208-215, https://doi.org/10.1016/s0166-2236(98)01349-6.
Harada, K., Kamiya, T., and Tsuboi, T. (2016) Gliotransmitter release from astrocytes: functional, developmental, and pathological implications in the brain, Front. Neurosci., 9, 499, https://doi.org/10.3389/fnins.2015.00499.
Haydon, P. G., and Carmignoto, G. (2006) Astrocyte control of synaptic transmission and neurovascular coupling, Physiol. Rev., 86, 1009-1031, https://doi.org/10.1152/physrev.00049.2005.
Fellin, T., and Carmignoto, G. (2004) Neurone-to-astrocyte signalling in the brain represents a distinct multifunctional unit, J. Physiol., 559 (Pt 1), 3-15, https://doi.org/10.1113/jphysiol.2004.063214.
Hamilton, N. B., and Attwell, D. (2010) Do astrocytes really exocytose neurotransmitters?, Nat. Rev. Neurosci., 11, 227-238, https://doi.org/10.1038/nrn2803.
Petrelli, F., and Bezzi, P. (2016) Novel insights into gliotransmitters, Curr. Opin. Pharmacol., 26, 138-145, https://doi.org/10.1016/j.coph.2015.11.010.
Baldwin, K. T., and Eroglu, C. (2017) Molecular mechanisms of astrocyte-induced synaptogenesis, Curr. Opin. Neurobiol., 45, 113-120, https://doi.org/10.1016/j.conb.2017.05.006.
Barber, C. N., and Raben, D. M. (2019) Lipid metabolism crosstalk in the brain: glia and neurons, Front. Cell Neurosci., 13, 212, https://doi.org/10.3389/fncel.2019.00212.
Semyanov, A., and Verkhratsky, A. (2021) Astrocytic processes: from tripartite synapses to the active milieu, Trends Neurosci., 44, 781-792, https://doi.org/10.1016/j.tins.2021.07.006.
Fitzner, D., Bader, J. M., Penkert, H., Bergner, C. G., Su, M., Weil, M. T., Surma, M. A., Mann, M., Klose, C., and Simons, M. (2020) Cell-type- and brain-region-resolved mouse brain lipidome, Cell Rep., 32, 108132, https://doi.org/10.1016/j.celrep.2020.108132.
Galkina, O. V., Putilina, F. E., and Eshchenko, N. D. (2014) Changes in the lipid composition of the brain during early onthogenesis, Neurochem. J., 8, 83-88, https://doi.org/10.1134/S1819712414020044.
Lee, J. A., Hall, B., Allsop, J., Alqarni, R., and Allen, S. P. (2021) Lipid metabolism in astrocytic structure and function, Semin. Cell Dev. Biol., 112, 123-136, https://doi.org/10.1016/j.semcdb.2020.07.017.
Zhu, Y. B., Gao, W., Zhang, Y., Jia, F., Zhang, H. L., Liu, Y. Z., Sun, X. F., Yin, Y., and Yin, D. M. (2016) Astrocyte-derived phosphatidic acid promotes dendritic branching, Sci. Rep., 6, 21096, https://doi.org/10.1038/srep21096.
Cai, D., Zhong, M., Wang, R., Netzer, W.J., Shields, D., Zheng, H., Sisodia, S. S., Foster, D. A., Gorelick, F. S., Xu, H., and Greengard, P. (2006) Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer’s disease-linked presenilin-1 mutant neurons, Proc. Nat. Acad. Sci. USA, 103, 1936-1940, https://doi.org/10.1073/pnas.0510710103.
Tanguy, E., Wang, Q., Moine, H., and Vitale, N. (2019) Phosphatidic acid: from pleiotropic functions to neuronal pathology, Front. Cell Neurosci., 13, 2, https://doi.org/10.3389/fncel.2019.00002.
Tabet, R., Moutin, E., Becker, J. A., Heintz, D., Fouillen, L., Flatter, E., Krężel, W., Alunni, V., Koebel, P., Dembélé, D., Tassone, F., Bardoni, B., Mandel, J. L., Vitale, N., Muller, D., Le Merrer, J., and Moine, H. (2016) Fragile X Mental Retardation Protein (FMRP) controls diacylglycerol kinase activity in neurons, Proc. Natl. Acad. Sci. USA, 113, E3619-E3628, https://doi.org/10.1073/pnas.1522631113.
Huang, P., Altshuller, Y. M., Hou, J. C., Pessin, J. E., and Frohman, M. A. (2005) Insulin-stimulated plasma membrane fusion of Glut4 glucose transporter-containing vesicles is regulated by phospholipase D1, Mol. Biol. Cell, 16, 2614-2623, https://doi.org/10.1091/mbc.e04-12-1124.
Shirai, Y., and Saito, N. (2014) Diacylglycerol kinase as a possible therapeutic target for neuronal diseases, J. Biomed. Sci., 21, 28, https://doi.org/10.1186/1423-0127-21-28.
Lee, D., Kim, E., Tanaka-Yamamoto, K. (2016) Diacylglycerol kinases in the coordination of synaptic plasticity, Front. Cell Dev. Biol., 4, 92, https://doi.org/10.3389/fcell.2016.00092.
Barber, C. N., and Raben, D. M. (2020) Roles of DGKs in neurons: postsynaptic functions?, Adv. Biol. Regul., 75, 100688, https://doi.org/10.1016/j.jbior.2019.100688.
Hozumi, Y., Watanabe, M., Otani, K., and Goto, K. (2009) Diacylglycerol kinase beta promotes dendritic outgrowth and spine maturation in developing hippocampal neurons, BMC Neurosci., 10, 99, https://doi.org/10.1186/1471-2202-10-99.
Shirai, Y., Kouzuki, T., Kakefuda, K., Moriguchi, S., Oyagi, A., Horie, K., Morita, S. Y., Shimazawa, M., Fukunaga, K., Takeda, J., Saito, N., and Hara, H. (2010) Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function, PLoS One, 5, e11602, https://doi.org/10.1371/journal.pone.0011602.
Seo, J., Kim, K., Jang, S., Han, S., Choi, S. Y., and Kim, E. (2012) Regulation of hippocampal long-term potentiation and long-term depression by diacylglycerol kinase ζ, Hippocampus, 22, 1018-1026, https://doi.org/10.1002/hipo.20889.
Goto, K., Watanabe, M., Kondo, H., Yuasa, H., Sakane, F., and Kanoh, H. (1992) Gene cloning, sequence, expression and in situ localization of 80 kDa diacylglycerol kinase specific to oligodendrocyte of rat brain, Brain Res. Mol. Brain Res., 16, 75-87, https://doi.org/10.1016/0169-328x(92)90196-i.
Wheeler, S. E., Stacey, H. M., Nahaei, Y., Hale, S. J., Hardy, A. B., Reimann, F., Gribble, F. M., Larraufie, P., Gaisano, H. Y., and Brubaker, P. L. (2017) The SNARE protein syntaxin-1a plays an essential role in biphasic exocytosis of the incretin hormone glucagon-like peptide 1, Diabetes, 66, 2327-2338, https://doi.org/10.2337/db16-1403.
Tanguy, E., Kassas, N., and Vitale, N. (2018) Protein-phospholipid interaction motifs: a focus on phosphatidic acid, Biomolecules, 8, 20, https://doi.org/10.3390/biom8020020.
Limatola, C., Schaap, D., Moolenaar, W. H., and van Blitterswijk, W. J. (1994) Phosphatidic acid activation of protein kinase C-zeta overexpressed in COS cells: comparison with other protein kinase C isotypes and other acidic lipids, Biochem. J., 304 (Pt 3), 1001-1008, https://doi.org/10.1042/bj3041001.
Jang, J.-H., Lee, C. S., Hwang, D., and Ryu, S. H. (2012) Understanding of the roles of phospholipase D and phosphatidic acid through their binding partners, Prog. Lipid Res., 51, 71-81, https://doi.org/10.1016/j.plipres.2011.12.003.
Park, C., Kang, D. S., Shin, G. H., Seo, J., Kim, H., Suh, P. G., Bae, C. D., and Shin, J. H. (2015) Identification of novel phosphatidic acid-binding proteins in the rat brain, Neurosci. Lett., 595, 108-113, https://doi.org/10.1016/j.neulet.2015.04.012.
Kassas, N., Tanguy, E., Thahouly, T., Fouillen, L., Heintz, D., Chasserot-Golaz, S., Bader, M. F., Grant, N. J., and Vitale, N. (2017) Comparative characterization of phosphatidic acid sensors and their localization during frustrated phagocytosis, J. Biol. Chem., 292, 4266-4279, https://doi.org/10.1074/jbc.M116.742346.
Sang, N., Zhang, J., Marcheselli, V., Bazan, N. G., and Chen, C. (2005) Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor, J. Neurosci., 25, 9858-9870, https://doi.org/10.1523/JNEUROSCI.2392-05.2005.
Lima, I. V., Bastos, L. F., Limborço-Filho, M., Fiebich, B. L., and de Oliveira, A. C. (2012) Role of prostaglandins in neuroinflammatory and neurodegenerative diseases, Mediators Inflamm., 2012, 946813, https://doi.org/10.1155/2012/946813.
Pedersen, A. L., and Saldanha, C. J. (2017) Reciprocal interactions between prostaglandin E2- and estradiol-dependent signaling pathways in the injured zebra finch brain, J. Neuroinflamm., 14, 262, https://doi.org/10.1186/s12974-017-1040-1.
Attwell, D., Buchan, A. M., Charpak, S., Lauritzen, M., Macvicar, B. A., and Newman, E. A. (2010) Glial and neuronal control of brain blood flow, Nature, 468, 232-243, https://doi.org/10.1038/nature09613.
Liu, Y., Zhang, H., Wu, C. Y., Yu, T., Fang, X., Ryu, J. J., Zheng, B., Chen, Z., Roman, R. J., and Fan, F. (2021) 20-HETE-promoted cerebral blood flow autoregulation is associated with enhanced pericyte contractility, Prostaglandins Other Lipid Mediat., 154, 106548, https://doi.org/10.1016/j.prostaglandins.2021.106548.
Navarrete, M., Perea, G., Maglio, L., Pastor, J., García de Sola, R., and Araque, A. (2013) Astrocyte calcium signal and gliotransmission in human brain tissue, Cereb. Cortex, 23, 1240-1246, https://doi.org/10.1093/cercor/bhs122.
Navarrete, M., Díez, A., and Araque, A. (2014) Astrocytes in endocannabinoid signalling, Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci., 369, 20130599, https://doi.org/10.1098/rstb.2013.0599.
Rouzer, C. A., and Marnett, L. J. (2011) Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways, Chem. Rev., 111, 5899-5921, https://doi.org/10.1021/cr2002799.
Mergenthaler, P., Lindauer, U., Dienel, G. A., and Meisel, A. (2013) Sugar for the brain: the role of glucose in physiological and pathological brain function, Trends Neurosci., 36, 587-597, https://doi.org/10.1016/j.tins.2013.07.001.
Falkowska, A., Gutowska, I., Goschorska, M., Nowacki, P., Chlubek, D., and Baranowska-Bosiacka, I. (2015) Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism, Int. J. Mol. Sci., 16, 25959-25981, https://doi.org/10.3390/ijms161125939.
Furuya, S.T., Tabata, J., Mitoma, K., Yamada, M., Yamasaki, A., Makino, A., Yamamoto, T., Watanabe, M., Kano, M., and Hirabayashi, Y. (2000) L-Serine and glycine serve as major astroglia-derived trophic factors for cerebellar Purkinje neurons, Proc. Natl. Acad. Sci. USA, 97, 11528-11533, https://doi.org/10.1073/pnas.200364497.
Chen, J., Zhang, X., Kusumo, H., Costa, L. G., and Guizzetti, M. (2013) Cholesterol efflux is differentially regulated in neurons and astrocytes: Implications for brain cholesterol homeostasis, Biochim. Biophys. Acta, 1831, 263-275, https://doi.org/10.1016/j.bbalip.2012.09.007.
Van Deijk, A. F., Camargo, N., Timmerman, J., Heistek, T., Brouwers, J. F., Mogavero, F., Mansvelder, H. D., Smit, A. B., and Verheijen, M. H. (2017) Astrocyte lipid metabolism is critical for synapse development and function in vivo, Glia, 65, 670-682, https://doi.org/10.1002/glia.23120.
McPherson, P.A., and McEneny, J. (2012) The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress, J. Physiol. Biochem., 68, 141-151, https://doi.org/10.1007/s13105-011-0112-4.
Schonfeld, P., and Reiser, G. (2013) Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain, J. Cereb. Blood Flow Metab., 33, 1493-1499, https://doi.org/10.1038/jcbfm.2013.128.
Speijer, D., Manjeri, G. R., and Szklarczyk, R. (2014) How to deal with oxygen radicals stemming from mitochondrial fatty acid oxidation, Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci., 369, 20130446, https://doi.org/10.1098/rstb.2013.0446.
Bailey, A. P., Koster, G., Guillermier, C., Hirst, E. M., MacRae, J. I., Lechene, C. P., Postle, A. D., and Gould, A. P. (2015) Antioxidant role for lipid droplets in a stem cell niche of Drosophila, Cell, 163, 340-353, https://doi.org/10.1016/j.cell.2015.09.020.
Smolič, T., Zorec, R., and Vardjan, N. (2021) Pathophysiology of lipid droplets in neuroglia, Antioxidants, 11, 22, https://doi.org/10.3390/antiox11010022.
Ioannou, M. S., Jackson, J., Sheu, S. H., Chang, C. L., Weigel, A. V., Liu, H., Pasolli, H. A., Xu, C. S., Pang, S., Matthies, D., Hess, H. F., Lippincott-Schwartz, J., and Liu, Z. (2019) Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity, Cell, 177, 1522-1535.e14, https://doi.org/10.1016/j.cell.2019.04.001.
Yang, D., Wang, X., Zhang, L., Fang, Y., Zheng, Q., Liu, X., Yu, W., Chen, S., Ying, J., and Hua, F. (2022) Lipid metabolism and storage in neuroglia: role in brain development and neurodegenerative diseases, Cell Biosci., 12, 106, https://doi.org/10.1186/s13578-022-00828-0.
Moore, S. A. (2001) Polyunsaturated fatty acid synthesis and release by brain-derived cells in vitro, J. Mol. Neurosci., 16, 195-200, https://doi.org/10.1385/JMN:16:2-3:195.
Nieweg, K., Schaller, H., and Pfrieger, F. W. (2009) Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats, J. Neurochem., 109, 125-134, https://doi.org/10.1111/j.1471-4159.2009.05917.x.
Garcia Corrales, A. V., Haidar, M., Bogie, J. F. J., and Hendriks, J. J. A. (2021) Fatty acid synthesis in glial cells of the CNS, Int. J. Mol. Sci., 22, 8159, https://doi.org/10.3390/ijms22158159.
Aizawa, F., Nishinaka, T., Yamashita, T., Nakamoto, K., Koyama, Y., Kasuya, F., and Tokuyama, S. (2016) Astrocytes release polyunsaturated fatty acids by lipopolysaccharide stimuli, Biol. Pharm. Bull., 39, 1100-1106, https://doi.org/10.1248/bpb.b15-01037.
Pfrieger, F. W., and Ungerer, N. (2011) Cholesterol metabolism in neurons and astrocytes, Progr. Lipid Res., 50, 357-371, https://doi.org/10.1016/j.plipres.2011.06.002.
Orth, M., and Bellosta, S. (2012) Cholesterol: its regulation and role in central nervous system disorders, Cholesterol, 2012, 292598, https://doi.org/10.1155/2012/292598.
Zhang, J., and Liu, Q. (2015) Cholesterol metabolism and homeostasis in the brain, Protein Cell, 6, 254-264, https://doi.org/10.1007/s13238-014-0131-3.
Dietschy, J. M., and Turley, S. D. (2004) Cholesterol metabolism in the central nervous system during early development and in the mature animal, J. Lipid Res., 45, 1375-1397, https://doi.org/10.1194/jlr.R400004-JLR200.
Mauch, D. H., Nägler, K., Schumacher, S., Göritz, C., Müller, E. C., Otto, A., and Pfrieger, F. W. (2001) CNS synaptogenesis promoted by glia-derived cholesterol, Science, 294, 1354-1357, https://doi.org/10.1126/science.294.5545.1354.
Göritz, C., Mauch, D. H., Nägler, K., and Pfrieger, F. W. (2002) Role of glia-derived cholesterol in synaptogenesis: new revelations in the synapse-glia affair, J. Physiol. Paris, 96, 257-263, https://doi.org/10.1016/s0928-4257(02)00014-1.
Moutinho, M., Nunes, M. J., and Rodrigues, E. (2017) The mevalonate pathway in neurons: It’s not just about cholesterol, Exp. Cell Res., 360, 55-60, https://doi.org/10.1016/j.yexcr.2017.02.034.
Lloyd-Evans, E., and Waller-Evans, H. (2020) Biosynthesis and signalling functions of central and peripheral nervous system neurosteroids in health and disease, Essays Biochem., 64, 591-606, https://doi.org/10.1042/EBC20200043.
Camargo, N., Brouwers, J. F., Loos, M., Gutmann, D. H., Smit, A. B., and Verheijen, M. H. (2012) High-fat diet ameliorates neurological deficits caused by defective astrocyte lipid metabolism, FASEB J., 26, 4302-4315, https://doi.org/10.1096/fj.12-205807.
Thiele, C., Hannah, M. J., Fahrenholz, F., and Huttner, W. B. (2000) Cholesterol binds to synaptophysin and is required for biogenesis of synaptic vesicles, Nat. Cell Biol., 2, 42-49, https://doi.org/10.1038/71366.
Petrov, A. M., Kasimov, M. R., and Zefirov, A. L. (2016) Brain cholesterol metabolism and its defects: linkage to neurodegenerative diseases and synaptic dysfunction, Acta Naturae, 8, 58-73, https://doi.org/10.32607/20758251-2016-8-1-58-73.
Allen, J. A., Halverson-Tamboli, R. A., and Rasenick, M. M. (2007) Lipid raft microdomains and neurotransmitter signalling, Nat. Rev. Neurosci., 8, 128-140, https://doi.org/10.1038/nrn2059.
Delle Bovi, R. J., Kim, J., Suresh, P., London, E., and Miller, W. T. (2019) Sterol structure dependence of insulin receptor and insulin-like growth factor 1 receptor activation, Biochim. Biophys. Acta Biomembr., 1861, 819-826, https://doi.org/10.1016/j.bbamem.2019.01.009.
Tracey, T. J., Steyn, F. J., Wolvetang, E. J., and Ngo, S. T. (2018) Neuronal lipid metabolism: multiple pathways driving functional outcomes in health and disease, Front. Mol. Neurosci., 11, 10, https://doi.org/10.3389/fnmol.2018.00010.
Bruce, K. D., Zsombok, A., and Eckel, R. H. (2017) Lipid processing in the brain: a key regulator of systemic metabolism, Front. Endocrinol., 8, 60, https://doi.org/10.3389/fendo.2017.00060.
Killoy, K. M., Harlan, B. A., Pehar, M., and Vargas, M. R. (2020) FABP7 upregulation induces a neurotoxic phenotype in astrocytes, Glia, 68, 2693-2704, https://doi.org/10.1002/glia.23879.
Kagawa, Y., Yasumoto, Y., Sharifi, K., Ebrahimi, M., Islam, A., Miyazaki, H., Yamamoto, Y., Sawada, T., Kishi, H., Kobayashi, S., Maekawa, M., Yoshikawa, T., Takaki, E., Nakai, A., Kogo, H., Fujimoto, T., and Owada, Y. (2015) Fatty acid-binding protein 7 regulates function of caveolae in astrocytes through expression of caveolin-1, Glia, 63, 780-794, https://doi.org/10.1002/glia.22784.
Ebrahimi, M., Yamamoto, Y., Sharifi, K., Kida, H., Kagawa, Y., Yasumoto, Y., Islam, A., Miyazaki, H., Shimamoto, C., Maekawa, M., Mitsushima, D., Yoshikawa, T., and Owada, Y. (2016) Astrocyte-expressed FABP7 regulates dendritic morphology and excitatory synaptic function of cortical neurons, Glia, 64, 48-62, https://doi.org/10.1002/glia.22902.
Liu, L., MacKenzie, K. R., Putluri, N., Maletić-Savatić, M., and Bellen, H. J. (2017) The glia-neuron lactate shuttle and elevated ros promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D, Cell Metab., 26, 719-737.e6, https://doi.org/10.1016/j.cmet.2017.08.024.
Wang, H., and Eckel, R. H. (2014) What are lipoproteins doing in the brain?, Trends Endocrinol. Metab., 25, 8-14, https://doi.org/10.1016/j.tem.2013.10.003.
Evola, M., Hall, A., Wall, T., Young, A., and Grammas, P. (2010) Oxidative stress impairs learning and memory in apoE knockout mice, Pharm. Biochem. Behav., 96, 181-186, https://doi.org/10.1016/j.pbb.2010.05.003.
Weeber, E. J., Beffert, U., Jones, C., Christian, J. M., Forster, E., Sweatt, J. D., and Herz, J. (2002) Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning, J. Biol. Chem., 277, 39944-39952, https://doi.org/10.1074/jbc.M205147200.
Castellano, J. M., Kim, J., Stewart, F. R., Jiang, H., DeMattos, R. B., Patterson, B. W., Fagan, A. M., Morris, J. C., Mawuenyega, K. G., Cruchaga, C., Goate, A. M., Bales, K. R., Paul, S. M., Bateman, R. J., and Holtzman, D. M. (2011) Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance, Sci. Transl. Med., 3, 89ra57, https://doi.org/10.1126/scitranslmed.3002156.
Jones, P. B., Adams, K. W., Rozkalne, A., Spires-Jones, T. L., Hshieh, T. T., Hashimoto, T., von Armin, C. A., Mielke, M., Bacskai, B. J., and Hyman, B. T. (2011) Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-beta in human Alzheimer brain, PLoS One, 6, e14586, https://doi.org/10.1371/journal.pone.0014586.
Yamazaki, Y., Zhao, N., Caulfield, T. R., Liu, C. C., and Bu, G. (2019) Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies, Nat. Rev. Neurol., 15, 501-518, https://doi.org/10.1038/s41582-019-0228-7.
Yin, J., Spillman, E., Cheng, E. S., Short, J., Chen, Y., Lei, J., Gibbs, M., Rosenthal, J. S., Sheng, C., Chen, Y. X., Veerasammy, K., Choetso, T., Abzalimov, R., Wang, B., Han, C., He, Y., and Yuan, Q. (2021) Brain-specific lipoprotein receptors interact with astrocyte derived apolipoprotein and mediate neuron-glia lipid shuttling, Nat. Commun., 12, 2408, https://doi.org/10.1038/s41467-021-22751-7.
Cartocci, V., Servadio, M., Trezza, V., and Pallottini, V. (2017) Can cholesterol metabolism modulation affect brain function and behavior?, J. Cell Physiol., 232, 281-286, https://doi.org/10.1002/jcp.25488.
Alecu, I., and Bennett, S. (2019) Dysregulated lipid metabolism and its role in α-synucleinopathy in Parkinson’s disease, Front. Neurosci., 13, 328, https://doi.org/10.3389/fnins.2019.00328.
Adibhatla, R. M., and Hatcher, J. F. (2007) Role of lipids in brain injury and diseases, Fut. Lipidol., 2, 403-422, https://doi.org/10.2217/17460875.2.4.403.
Nave, K. A. (2010) Myelination and the trophic support of long axons, Nat. Rev. Neurosci., 11, 275-283, https://doi.org/10.1038/nrn2797.
Butt, A. M., Papanikolaou, M., and Rivera, A. (2019) Physiology of oligodendroglia, Adv. Exp. Med. Biol., 1175, 117-128, https://doi.org/10.1007/978-981-13-9913-8_5.
Sakry, D., Karram, K., and Trotter, J. (2011) Synapses between NG2 glia and neurons, J. Anat., 219, 2-7, https://doi.org/10.1111/j.1469-7580.2011.01359.x.
Papanikolaou, M., Butt, A. M., and Lewis, A. (2020) A critical role for the inward rectifying potassium channel Kir7.1 in oligodendrocytes of the mouse optic nerve, Brain Struct. Funct., 225, 925-934, https://doi.org/10.1007/s00429-020-02043-4.
Cherchi, F., Bulli, I., Venturini, M., Pugliese, A. M., and Coppi, E. (2021) Ion channels as new attractive targets to improve re-myelination processes in the brain, Int. J. Mol. Sci., 22, 7277, https://doi.org/10.3390/ijms22147277.
Gopalakrishnan, G., Awasthi, A., Belkaid, W., De Faria, O. Jr, Liazoghli, D., Colman, D. R., and Dhaunchak, A. S. (2013) Lipidome and proteome map of myelin membranes, J. Neurosci. Res., 91, 321-334, https://doi.org/10.1002/jnr.23157.
Löhmann, C., Schachmann, E., Dandekar, T., Villmann, C., and Becker, C. M. (2010) Developmental profiling by mass spectrometry of phosphocholine containing phospholipids in the rat nervous system reveals temporo-spatial gradients, J. Neurochem., 114, 1119-1134, https://doi.org/10.1111/j.1471-4159.2010.06836.x.
Montani, L. (2021) Lipids in regulating oligodendrocyte structure and function, Semin. Cell Dev. Biol., 112, 114-122, https://doi.org/10.1016/j.semcdb.2020.07.016.
Kassmann, C. M. (2014) Myelin peroxisomes – essential organelles for the maintenance of white matter in the nervous system, Biochimie, 98, 111-118, https://doi.org/10.1016/j.biochi.2013.09.020.
Singhal, N. K., Huang, H., Li, S., Clements, R., Gadd, J., Daniels, A., Kooijman, E. E., Bannerman, P., Burns, T., Guo, F., Pleasure, D., Freeman, E., Shriver, L., and McDonough, J. (2017) The neuronal metabolite NAA regulates histone H3 methylation in oligodendrocytes and myelin lipid composition, Exp. Brain Res., 235, 279-292, https://doi.org/10.1007/s00221-016-4789-z.
Saher, G., Brügger, B., Lappe-Siefke, C., Möbius, W., Tozawa, R., Wehr, M. C., Wieland, F., Ishibashi, S., and Nave, K. A. (2005) High cholesterol level is essential for myelin membrane growth, Nat. Neurosci., 8, 468-475, https://doi.org/10.1038/nn1426.
Saher, G., and Stumpf, S. K. (2015) Cholesterol in myelin biogenesis and hypomyelinating disorders, Biochim. Biophys. Acta, 1851, 1083-1094, https://doi.org/10.1016/j.bbalip.2015.02.010.
Mathews, E. S., Mawdsley, D. J., Walker, M., Hines, J. H., Pozzoli, M., and Appel, B. (2014) Mutation of 3-hydroxy-3-methylglutaryl CoA synthase I reveals requirements for isoprenoid and cholesterol synthesis in oligodendrocyte migration arrest, axon wrapping, and myelin gene expression, J. Neurosci., 34, 3402-3412, https://doi.org/10.1523/JNEUROSCI.4587-13.2014.
Camargo, N., Goudriaan, A., van Deijk, A. F., Otte, W. M., Brouwers, J. F., Lodder, H., Gutmann, D. H., Nave, K. A., Dijkhuizen, R. M., Mansvelder, H. D., Chrast, R., Smit, A. B., and Verheijen, M. H. G. (2017) Oligodendroglial myelination requires astrocyte-derived lipids, PLoS Biol., 15, e1002605, https://doi.org/10.1371/journal.pbio.1002605.
Yu, T., and Lieberman, A. P. (2013) Npc1 acting in neurons and glia is essential for the formation and maintenance of CNS myelin, PLoS Genet., 9, e1003462, https://doi.org/10.1371/journal.pgen.1003462.
Mathews, E. S., and Appel, B. (2016) Cholesterol biosynthesis supports myelin gene expression and axon ensheathment through modulation of P13K/Akt/mTor signaling, J. Neurosci., 36, 7628-7639, https://doi.org/10.1523/JNEUROSCI.0726-16.2016.
Posse de Chaves, E., and Sipione, S. (2010) Sphingolipids and gangliosides of the nervous system in membrane function and dysfunction, FEBS Lett., 584, 1748-1759, https://doi.org/10.1016/j.febslet.2009.12.010.
Bonetto, G., and Di Scala, C. (2019) Importance of lipids for nervous system integrity: cooperation between gangliosides and sulfatides in myelin stability, J. Neurosci., 39, 6218-6220, https://doi.org/10.1523/JNEUROSCI.0377-19.2019.
Susuki, K., Baba, H., Tohyama, K., Kanai, K., Kuwabara, S., Hirata, K., Furukawa, K., Furukawa, K., Rasband, M. N., and Yuki, N. (2007) Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers, Glia, 55, 746-757, https://doi.org/10.1002/glia.20503.
Ishibashi, T., Dupree, J. L., Ikenaka, K., Hirahara, Y., Honke, K., Peles, E., Popko, B., Suzuki, K., Nishino, H., and Baba, H. (2002) A myelin galactolipid, sulfatide, is essential for maintenance of ion channels on myelinated axon but not essential for initial cluster formation, J. Neurosci., 22, 6507-6514, https://doi.org/10.1523/JNEUROSCI.22-15-06507.2002.
Yang, L. J., Zeller, C. B., Shaper, N. L., Kiso, M., Hasegawa, A., Shapiro, R. E., and Schnaar, R. L. (1996) Gangliosides are neuronal ligands for myelin-associated glycoprotein, Proc. Natl. Acad. Sci. USA, 93, 814-818, https://doi.org/10.1073/pnas.93.2.814.
Vinson, M., Strijbos, P. J., Rowles, A., Facci, L., Moore, S. E., Simmons, D. L., and Walsh, F. S. (2001) Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition, J. Biol. Chem., 276, 20280-20285, https://doi.org/10.1074/jbc.M100345200.
Pronker, M., Lemstra, S., Snijder, J., Heck, A. J., Thies-Weesie, D. M., Pasterkamp, R. J., and Janssen, B. J. (2016) Structural basis of myelin-associated glycoprotein adhesion and signalling, Nat. Commun., 7, 13584, https://doi.org/10.1038/ncomms13584.
Kasahara, K., Watanabe, K., Takeuchi, K., Kaneko, H., Oohira, A., Yamamoto, T., and Sanai, Y. (2000) Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts, J. Biol. Chem., 275, 34701-34709, https://doi.org/10.1074/jbc.M003163200.
Loberto, N., Prioni, S., Prinetti, A., Ottico, E., Chigorno, V., Karagogeos, D., and Sonnino, S. (2003) The adhesion protein TAG-1 has a ganglioside environment in the sphingolipid-enriched membrane domains of neuronal cells in culture, J. Neurochem., 85, 224-233, https://doi.org/10.1046/j.1471-4159.2003.01655.x.
Hammond, T. R., Dufort, C., Dissing-Olesen, L., Giera, S., Young, A., Wysoker, A., Walker, A. J., Gergits, F., Segel, M., Nemesh, J., Marsh, S. E., Saunders, A., Macosko, E., Ginhoux, F., Chen, J., Franklin, R. J. M., Piao, X., McCarroll, S. A., and Stevens, B. (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes, Immunity, 50, 253-271.e6, https://doi.org/10.1016/j.immuni.2018.11.004.
Li, Q., and Barres, B. (2018) Microglia and macrophages in brain homeostasis and disease, Nat. Rev. Immunol., 18, 225-242, https://doi.org/10.1038/nri.2017.125.
Loving, B. A., and Bruce, K. D. (2020) Lipid and lipoprotein metabolism in microglia, Front. Physiol., 11, 393, https://doi.org/10.3389/fphys.2020.00393.
Lombardi, M., Parolisi, R., Scaroni, F., Bonfanti, E., Gualerzi, A., Gabrielli, M., Kerlero de Rosbo, N., Uccelli, A., Giussani, P., Viani, P., Garlanda, C., Abbracchio, M. P., Chaabane, L., Buffo, A., Fumagalli, M., and Verderio, C. (2019) Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure, Acta Neuropathol., 138, 987-1012, https://doi.org/10.1007/s00401-019-02049-1.
Folick, A., Koliwad, S. K., and Valdearcos, M. (2021) Microglial lipid biology in the hypothalamic regulation of metabolic homeostasis, Front. Endocrinol. (Lausanne), 12, 668396, https://doi.org/10.3389/fendo.2021.668396.
Zhan, Y., Paolicelli, R. C., Sforazzini, F., Weinhard, L., Bolasco, G., Pagani, F., Vyssotski, A. L., Bifone, A., Gozzi, A., Ragozzino, D., and Gross, C. T. (2014) Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior, Nat. Neurosci., 17, 400-406, https://doi.org/10.1038/nn.3641.
Chausse, B., Kakimoto, P. A., and Kann, O. (2021) Microglia and lipids: how metabolism controls brain innate immunity, Semin. Cell Dev. Biol., 112, 137-144, https://doi.org/10.1016/j.semcdb.2020.08.001.
Chausse, B., Kakimoto, P. A., Caldeira-da-Silva, C. C., Chaves-Filho, A. B., Yoshinaga, M. Y., Yoshinaga, M. Y., da Silva, R. P., Miyamoto, S., and Kowaltowski, A. J. (2019) Distinct metabolic patterns during microglial remodeling by oleate and palmitate, Biosci. Rep., 39, BSR20190072, https://doi.org/10.1042/BSR20190072.
Bohlen, C. J., Bennett, F. C., Tucker, A. F., Collins, H. Y., Mulinyawe, S. B., and Barres, B. A. (2017) Diverse requirements for microglial survival, specification, and function revealed by defined-medium cultures, Neuron, 94, 759-773.e8, https://doi.org/10.1016/j.neuron.2017.04.043.
Kopper, T. J., and Gensel, J. C. (2018) Myelin as an inflammatory mediator: myelin interactions with complement, macrophages, and microglia in spinal cord injury, J. Neurosci. Res., 96, 969-977, https://doi.org/10.1002/jnr.24114.
Grajchen, E., Wouters, E., van de Haterd, B., Haidar, M., Hardonnière, K., Dierckx, T., Van Broeckhoven, J., Erens, C., Hendrix, S., Kerdine-Römer, S., Hendriks, J. J. A., and Bogie, J. F. J. (2020) CD36-mediated uptake of myelin debris by macrophages and microglia reduces neuroinflammation, J. Neuroinflammation, 17, 224, https://doi.org/10.1186/s12974-020-01899-x.
Cantuti-Castelvetri, L., Fitzner, D., Bosch-Queralt, M., Weil, M.-T., Su, M., and Sen, P. (2018) Defective cholesterol clearance limits remyelination in the aged central nervous system, Science, 359, 684, https://doi.org/10.1126/science.aan4183.
Berghoff, S.A., Spieth, L., Sun, T., Hosang, L., Schlaphoff, L., Depp, C., Düking, T., Winchenbach, J., Neuber, J., Ewers, D., Scholz, P., van der Meer, F., Cantuti-Castelvetri, L., Sasmita, A. O., Meschkat, M., Ruhwedel, T., Möbius, W., Sankowski, R., Prinz, M., Huitinga, I., Sereda, M.W., Odoardi, F., Ischebeck, T., Simons, M., Stadelmann-Nessler, C., Edgar, J.M., Nave, K.A., and Saher, G. (2021) Microglia facilitate repair of demyelinated lesions via post-squalene sterol synthesis, Nat. Neurosci., 24, 47-60, https://doi.org/10.1038/s41593-020-00757-6.
Leyrolle, Q., Layé, S., and Nadjar, A. (2019) Direct and indirect effects of lipids on microglia function, Neurosci. Lett., 708, 134348, https://doi.org/10.1016/j.neulet.2019.134348.
Funding
The study was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement no. 075-15-2020-921, dated 13.11.2020) within the framework of the project of the NCMU Pavlovsk Center “Integrative Physiology – Medicine, High-Tech Healthcare and Stress Resistance Technologies”, direction: “Biological and Social Foundations of Inclusion”.
Author information
Authors and Affiliations
Contributions
O. V. Galkina, O. V. Vetrovoy, I. E. Krasovskaya, N. D. Eschenko – wrote the manuscript; O. V. Galkina – analyzed publications; N. D. Eschenko, O. V. Vetrovoy – substantially contributed to the study concept and design; O. V. Galkina, O. V. Vetrovoy – prepared visual materials; I. E. Krasovskaya – edited the manuscript.
Corresponding author
Ethics declarations
Authors declare no conflict of interests in financial or any other sphere. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Galkina, O.V., Vetrovoy, O.V., Krasovskaya, I.E. et al. Role of Lipids in Regulation of Neuroglial Interactions. Biochemistry Moscow 88, 337–352 (2023). https://doi.org/10.1134/S0006297923030045
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923030045