Abstract
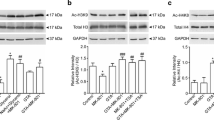
Organism adaptation to metabolic challenges requires coupling of metabolism to gene expression. In this regard, acylations of histones and metabolic proteins acquire significant interest. We hypothesize that adaptive response to inhibition of a key metabolic process, catalyzed by the acetyl-CoA-generating pyruvate dehydrogenase (PDH) complex, is mediated by changes in the protein acylations. The hypothesis is tested by intranasal administration to animals of PDH-specific inhibitors acetyl(methyl)phosphinate (AcMeP) or acetylphosphonate methyl ester (AcPMe), followed by the assessment of physiological parameters, brain protein acylation, and expression/phosphorylation of PDHA subunit. At the same dose, AcMeP, but not AcPMe, decreases acetylation and increases succinylation of the brain proteins with apparent molecular masses of 15-20 kDa. Regarding the proteins of 30-50 kDa, a strong inhibitor AcMeP affects acetylation only, while a less efficient AcPMe mostly increases succinylation. The unchanged succinylation of the 30-50 kDa proteins after the administration of AcMeP coincides with the upregulation of desuccinylase SIRT5. No significant differences between the levels of brain PDHA expression, PDHA phosphorylation, parameters of behavior or ECG are observed in the studied animal groups. The data indicate that the short-term inhibition of brain PDH affects acetylation and/or succinylation of the brain proteins, that depends on the inhibitor potency, protein molecular mass, and acylation type. The homeostatic nature of these changes is implied by the stability of physiological parameters after the PDH inhibition.










Similar content being viewed by others
Abbreviations
- MDH:
-
malate dehydrogenase
- OGDH:
-
2-oxoglutarate dehydrogenase (alpha-ketoglutarate dehydrogenase)
- PDH:
-
pyruvate dehydrogenase
- PDHA:
-
alpha subunit of PDH
- RMSSD:
-
the root mean square of successive differences in R-R intervals
- R-R interval:
-
the interval between the heartbeats
- SD:
-
the standard deviation of R-R interval
- SI:
-
stress index
References
Joseph, A. D. A., Robert, A. T., and Lawrence, R. G. (1980) Concentration-Dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein, Biochemistry, 19, 2656-2671, https://doi.org/10.1021/BI00553A019.
Fischle, W., Wang, Y., and Allis, C. D. (2003) Histone and chromatin cross-talk, Curr. Opin. Cell Biol., 15, 172-183, https://doi.org/10.1016/s0955-0674(03)00013-9.
Jeans, C., Thelen, M. P., and Noy, A. (2006) Single molecule studies of chromatin, Front. Cell Dev. Biol., 9, 699771, https://doi.org/10.2172/877892.
Akiyama, S. K., and Hammes, G. G. (1980) Elementary steps in the reaction mechanism of the pyruvate dehydrogenase multienzyme complex from Escherichia coli: kinetics of acetylation and deacetylation, Biochemistry, 19, 4208-4213, https://doi.org/10.1021/BI00559A011.
Waskiewicz, D. E., and Hammes, G. G. (1984) Elementary steps in the reaction mechanism of the α-ketoglutarate dehydrogenase multienzyme complex from Escherichia coli: kinetics of succinylation and desuccinylation, Biochemistry, 23, 3136-3143, https://doi.org/10.1021/BI00309A005.
Xu, Y., Shi, Z., and Bao, L. (2022) An expanding repertoire of protein acylations, Mol. Cell. Proteomics, 21, 100193, https://doi.org/10.1016/J.MCPRO.2022.100193.
Bruzzone, S., Parenti, M. D., Grozio, A., Ballestrero, A., Bauer, I., del Rio, A., and Nencioni, A. (2013) Rejuvenating sirtuins: the rise of a new family of cancer drug targets, Ingentaconnect. Com., 19, 614-623, https://doi.org/10.2174/138161213804581954.
Grabowska, W., Sikora, E., and Bielak-Zmijewska, A. (2017) Sirtuins, a promising target in slowing down the ageing process, Biogerontology, 18, 447-476, https://doi.org/10.1007/S10522-017-9685-9.
Sutendra, G., Kinnaird, A., Dromparis, P., Paulin, R., Stenson, T. H., Haromy, A., Hashimoto, K., Zhang, N., Flaim, E., and Michelakis, E. D. (2014) A nuclear pyruvate dehydrogenase complex is important for the generation of Acetyl-CoA and histone acetylation, Cell, 158, 84-97, https://doi.org/10.1016/J.CELL.2014.04.046.
Chueh, F. Y., Leong, K. F., Cronk, R. J., Venkitachalam, S., Pabich, S., and Yu, C. L. (2011) Nuclear localization of pyruvate dehydrogenase complex-E2 (PDC-E2), a mitochondrial enzyme, and its role in signal transducer and activator of transcription 5 (STAT5)-dependent gene transcription, Cell Signal., 23, 1170-1178, https://doi.org/10.1016/J.CELLSIG.2011.03.004.
Wang, Y., Guo, Y. R., Liu, K., Yin, Z., Liu, R., Xia, Y., Tan, L., Yang, P., Lee, J. H., Li, X. J., Hawke, D., Zheng, Y., Qian, X., Lyu, J., He, J., Xing, D., Tao, Y. J., and Lu, Z. (2017) KAT2A coupled with the α-KGDH complex acts as a histone H3 succinyltransferase, Nature, 552, 273-277, https://doi.org/10.1038/NATURE25003.
Choi, S., Pfleger, J., Jeon, Y. H., Yang, Z., He, M., Shin, H., Sayed, D., Astrof, S., and Abdellatif, M. (2019) Oxoglutarate dehydrogenase and acetyl-CoA acyltransferase 2 selectively associate with H2A. Z-occupied promoters and are required for histone modifications, Biochim. Biophys. Acta Gene Regul. Mech., 1862, 194436, https://doi.org/10.1016/J.BBAGRM.2019.194436.
Boyko, A. I., Karlina, I. S., Zavileyskiy, L. G., Aleshin, V. A., Artiukhov, A. V., Kaehne, T., Ksenofontov, A. L., Ryabov, S. I., Graf, A. V., Tramonti, A., and Bunik, V. I. (2022) Delayed impact of 2-oxoadipate dehydrogenase inhibition on the rat brain metabolism is linked to protein glutarylation, Front. Med., 9, 896263, https://doi.org/10.3389/fmed.2022.896263.
Gibson, G. E., Xu, H., Chen, H.-L., Chen, W., Denton, T. T., and Zhang, S. Z. (2015) Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines, J. Neurochem., 134, 86-96, https://doi.org/10.1111/jnc.13096.
Zavileyskiy, L. G., Aleshin, V. A., Kaehne, T., Karlina, I. S., Artiukhov, A. V., Maslova, M. V., Graf, A. V., and Bunik, V. I. (2022) The brain protein acylation system responds to seizures in the rat model of PTZ-induced epilepsy, Int. J. Mol. Sci., 23, 12302, https://doi.org/10.3390/ijms232012302.
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F., and Kroemer, G. (2015) Acetyl coenzyme A: a central metabolite and second messenger, Cell Metab., 21, 805-821, https://doi.org/10.1016/j.cmet.2015.05.014.
Mews, P., Donahue, G., Drake, A. M., Luczak, V., Abel, T., and Berger, S. L. (2017) Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory, Nature, 546, 381-386, https://doi.org/10.1038/NATURE22405.
Pougovkina, O., te Brinke, H., Ofman, R., van Cruchten, A. G., Kulik, W., Wanders, R. J. A., Houten, S. M., and de Boer, V. C. J. (2014) Mitochondrial protein acetylation is driven by acetyl-CoA from fatty acid oxidation, Hum. Mol. Genet., 23, 3513-3522, https://doi.org/10.1093/HMG/DDU059.
Gräff, J., and Tsai, L.-H. (2013) Histone acetylation: molecular mnemonics on the chromatin, Nat. Rev. Neurosci., 14, 97-111, https://doi.org/10.1038/nrn3427.
Gibson, G. E., Blass, J. P., Beal, M. F., and Bunik, V. (2005) The α-ketoglutarate-dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration, Mol. Neurobiol., 31, 43-63, https://doi.org/10.1385/MN:31:1-3:043.
Yang, Y., Tapias, V., Acosta, D., Xu, H., Chen, H., Bhawal, R., Anderson, E. T., Ivanova, E., Lin, H., Sagdullaev, B. T., Chen, J., Klein, W. L., Viola, K. L., Gandy, S., Haroutunian, V., Beal, M. F., Eliezer, D., Zhang, S., and Gibson, G. E. (2022) Altered succinylation of mitochondrial proteins, APP and tau in Alzheimer’s disease, Nat. Commun., 13, 159, https://doi.org/10.1038/S41467-021-27572-2.
Bunik, V. I., Tylicki, A., and Lukashev, N. V. (2013) Thiamin diphosphate‐dependent enzymes: from enzymology to metabolic regulation, drug design and disease models, FEBS J., 280, 6412-6442, https://doi.org/10.1111/febs.12512.
Bunik, V. I., Artiukhov, A., Kazantsev, A., Goncalves, R., Daloso, D., Oppermann, H., Kulakovskaya, E., Lukashev, N., Fernie, A., Brand, M., and Gaunitz, F. (2015) Specific inhibition by synthetic analogs of pyruvate reveals that the pyruvate dehydrogenase reaction is essential for metabolism and viability of glioblastoma cells, Oncotarget, 6, 40036-40052, https://doi.org/10.18632/ONCOTARGET.5486.
Nemeria, N. S., Korotchkina, L. G., Chakraborty, S., Patel, M. S., and Jordan, F. (2006) Acetylphosphinate is the most potent mechanism-based substrate-like inhibitor of both the human and Escherichia coli pyruvate dehydrogenase components of the pyruvate dehydrogenase complex, Bioorg. Chem., 34, 362-379, https://doi.org/10.1016/J.BIOORG.2006.09.001.
Artiukhov, A. V., Graf, A. V., and Bunik, V. I. (2016) Directed regulation of multienzyme complexes of 2-oxo acid dehydrogenases using phosphonate and phosphinate analogs of 2-oxo acids, Biochemistry (Moscow), 81, 1498-1521, https://doi.org/10.1134/S0006297916120129.
Bunik, V. I., and Fernie, A. R. (2009) Metabolic control exerted by the 2-oxoglutarate dehydrogenase reaction: a cross-kingdom comparison of the crossroad between energy production and nitrogen assimilation, Biochem. J., 422, 405-21, https://doi.org/10.1042/BJ20090722.
Artiukhov, A. V., Aleshin, V. A., Karlina, I. S., Kazantsev, A. v., Sibiryakina, D. A., Ksenofontov, A. L., Lukashev, N. V., Graf, A. V., and Bunik, V. I. (2022) Phosphonate inhibitors of pyruvate dehydrogenase perturb homeostasis of amino acids and protein succinylation in the brain, Int. J. Mol. Sci., 23, 13186, https://doi.org/10.3390/IJMS232113186.
Badhan, R. K. S., Kaur, M., Lungare, S., and Obuobi, S. (2014) Improving brain drug targeting through exploitation of the nose-to-brain route: a physiological and pharmacokinetic perspective, Curr. Drug Deliv., 11, 458-471, https://doi.org/10.2174/1567201811666140321113555.
Djupesland, P. G., Messina, J. C., and Mahmoud, R. A. (2014) The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview, Ther. Deliv., 5, 709-733, https://doi.org/10.4155/tde.14.41.
Pierozan, P., Jernerén, F., Ransome, Y., and Karlsson, O. (2017) The choice of euthanasia method affects metabolic serum biomarkers, Basic Clin. Pharmacol. Toxicol., 121, 113-118, https://doi.org/10.1111/BCPT.12774.
Suckow, M., Stevens, K., and Wilson, R. (2012) The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents, Academic Press.
Underwood, W., and Anthony, R. (2020) AVMA Guidelines for the Euthanasia of Animals: 2020 edition.
Aleshin, V. A., Graf, A. V., Artiukhov, A. V., Boyko, A. I., Ksenofontov, A. L., Maslova, M. V., Nogués, I., di Salvo, M. L., and Bunik, V. I. (2021) Physiological and biochemical markers of the sex-specific sensitivity to epileptogenic factors, delayed consequences of seizures and their response to vitamins B1 and B6 in rat model, Pharmaceuticals (Basel), 14, 737, https://doi.org/10.3390/ph14080737.
Jackson, H. F., and Broadhurst, P. L. (1982) The effects of Parachlorophenylalanine and stimulus intensity on open-field test measures in rats, Neuropharmacology, 21, 1279-1282, https://doi.org/10.1016/0028-3908(82)90133-2.
Liebsch, G., Montkowski, A., Holsboer, F., and Landgraf, R. (1998) Behavioral profiles of two Wistar rat lines selectively bred for high or low anxiety-related behaviour, Behavioural. Brain Res., 94, 301-310, https://doi.org/10.1016/S0166-4328(97)00198-8.
Aleshin, V. A., Mkrtchyan, G. V., Kaehne, T., Graf, A. V., Maslova, M. V., and Bunik, V. I. (2020) Diurnal regulation of the function of the rat brain glutamate dehydrogenase by acetylation and its dependence on thiamine administration, Wiley Online Library, 153, 80-102, https://doi.org/10.1111/jnc.14951.
Tsepkova, P., Artiukhov, A., Boyko, A., Aleshin, V. A., Mkrtchyan, G. V., Zvyagintseva, M. A., Ryabov, S. I., Ksenofontov, A. L., Baratova, L. A., Graf, A. V., and Bunik, V. I. (2017) Thiamine induces long-term changes in amino acid profiles and activities of 2-oxoglutarate and 2-oxoadipate dehydrogenases in rat brain, Biochemistry (Moscow), 82, 723-736, https://doi.org/10.1134/S0006297917060098.
Ladner, C. L., Yang, J., Turner, R. J., and Edwards, R. A. (2004) Visible fluorescent detection of proteins in polyacrylamide gels without staining, Anal. Biochem., 326, 13-20, https://doi.org/10.1016/J.AB.2003.10.047.
Kim, E. Y., Kim, W. K., Kang, H. J., Kim, J. H., Chung, S. J., Seo, Y. S., Park, S. G., Lee, S. C., and Bae, K. H. (2012) Acetylation of malate dehydrogenase 1 promotes adipogenic differentiation via activating its enzymatic activity, J. Lipid Res., 53, 1864-1876, https://doi.org/10.1194/JLR.M026567.
Tong, W. H., Maio, N., Zhang, D. L., Palmieri, E. M., Ollivierre, H., Ghosh, M. C., McVicar, D. W., and Rouault, T. A. (2018) TLR-activated repression of Fe-S cluster biogenesis drives a metabolic shift and alters histone and tubulin acetylation, Blood Adv, 2, 1146-1156, https://doi.org/10.1182/BLOODADVANCES.2018015669.
Hornbeck, P. V., Zhang, B., Murray, B., Kornhauser, J. M., Latham, V., and Skrzypek, E. (2015) PhosphoSitePlus, 2014: mutations, PTMs and recalibrations, Nucleic Acids Res., 43, D512-520, https://doi.org/10.1093/nar/gku1267.
Arumugam, R., Horowitz, E., Noland, R. C., Lu, D., Fleenor, D., and Freemark, M. (2010) Regulation of islet β-cell pyruvate metabolism: interactions of prolactin, glucose, and dexamethasone, Endocrinology, 151, 3074-3083, https://doi.org/10.1210/EN.2010-0049.
Consitt, L. A., Saxena, G., Saneda, A., and Houmard, J. A. (2016) Age-related impairments in skeletal muscle PDH phosphorylation and plasma lactate are indicative of metabolic inflexibility and the effects of exercise training, J. Physiol. Endocrinol. Metab., 311, E145-156, https://doi.org/10.1152/ajpendo.00452.2015.
Hossain, A. J., Islam, R., Kim, J.-G., Dogsom, O., Cap, K. C., and Park, J.-B. (2022) Pyruvate dehydrogenase A1 phosphorylated by insulin associates with pyruvate kinase M2 and induces LINC00273 through histone acetylation, Biomedicines, 10, 1256, https://doi.org/10.3390/BIOMEDICINES10061256.
Denton, R. M., McCormack, J. G., Rutter, G. A., Burnett, P., Edgell, N. J., Moule, S. K., and Diggle, T. A. (1996) The hormonal regulation of pyruvate dehydrogenase complex, Adv. Enzyme Regul., 36, 183-198, https://doi.org/10.1016/0065-2571(95)00020-8.
Kim, W., and Kaelin, J. W. G. (2003) The von Hippel-Lindau tumor suppressor protein: new insights into oxygen sensing and cancer, Curr. Opin. Genet Dev., 13, 55-60, https://doi.org/10.1016/S0959-437X(02)00010-2.
Li, X., Jiang, Y., Meisenhelder, J., Yang, W., Hawke, D. H., Zheng, Y., Xia, Y., Aldape, K., He, J., Hunter, T., Wang, L., and Lu, Z. (2016) Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis, Mol. Cell, 61, 705-719, https://doi.org/10.1016/J.MOLCEL.2016.02.009.
Nie, H., Ju, H., Fan, J., Shi, X., Cheng, Y., Cang, X., Zheng, Z., Duan, X., and Yi, W. (2020) O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth, Nat. Commun., 11, 36, https://doi.org/10.1038/S41467-019-13601-8.
Guan, J. S., Haggarty, S. J., Giacometti, E., Dannenberg, J. H., Joseph, N., Gao, J., Nieland, T. J. F., Zhou, Y., Wang, X., Mazitschek, R., Bradner, J. E., DePinho, R. A., Jaenisch, R., and Tsai, L. H. (2009) HDAC2 negatively regulates memory formation and synaptic plasticity, Nature, 459, 55-60, https://doi.org/10.1038/NATURE07925.
Li, X., Zhang, J., Li, D., He, C., He, K., Xue, T., Wan, L., Zhang, C., and Liu, Q. (2021) Astrocytic ApoE reprograms neuronal cholesterol metabolism and histone-acetylation-mediated memory, Neuron, 109, 957-970.e8, https://doi.org/10.1016/J.NEURON.2021.01.005.
Stilling, R. M., and Fischer, A. (2011) The role of histone acetylation in age-associated memory impairment and Alzheimer’s disease, Neurobiol. Learn Mem., 96, 19-26, https://doi.org/10.1016/J.NLM.2011.04.002.
Shields, G. S., Sazma, M. A., McCullough, A. M., and Yonelinas, A. P. (2017) The effects of acute stress on episodic memory: A meta-analysis and integrative review, Psychol. Bull., 143, 636-675, https://doi.org/10.1037/BUL0000100.
Goldfarb, E. V. (2019) Enhancing memory with stress: Progress, challenges, and opportunities, Brain Cogn., 133, 94-105, https://doi.org/10.1016/J.BANDC.2018.11.009.
Funding
This work was supported by the Russian Science Foundation (grant no. 18-14-00116 to V.I.B.).
Author information
Authors and Affiliations
Contributions
V.I.B developed the concept, supervised the study, wrote and edited the manuscript, obtained the funding, and administered the project. A.V.G. designed, supervised, and analyzed animal experiments; V.A.A. studied protein acylations, validated and visualized biochemical results; D.A.S. performed animal experiments and studied expression and phosphorylation of PDHA; A.V.K. provided the pyruvate analogs. All the co-authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
All animal experiments were performed according to the guidelines of the Declaration of Helsinki and were approved by Bioethics Committee of Lomonosov Moscow State University (protocol no. 139-a from 11 November 2021). The authors declare no conflict of interest. The funding sponsors had no role in the design of the study, collection, analysis, and interpretation of the data, writing of the manuscript, and decision to publish the results.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Aleshin, V.A., Sibiryakina, D.A., Kazantsev, A.V. et al. Acylation of the Rat Brain Proteins is Affected by the Inhibition of Pyruvate Dehydrogenase in vivo. Biochemistry Moscow 88, 105–118 (2023). https://doi.org/10.1134/S0006297923010091
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297923010091