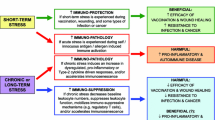
Abstract
Neurodegeneration involves progressive pathological loss of a specific population of neurons, glial activation, and dysfunction of myelinating oligodendrocytes leading to cognitive impairment and altered movement, breathing, and senses. Neuronal degeneration is a hallmark of aging, stroke, drug abuse, toxic chemical exposure, viral infection, chronic inflammation, and a variety of neurological diseases. Accumulation of intra- and extracellular protein aggregates is a common characteristic of cell pathologies. Excessive production of reactive oxygen species and nitric oxide, induction of endoplasmic reticulum stress, and accumulation of misfolded protein aggregates have been shown to trigger a defensive mechanism called integrated stress response (ISR). Activation of ISR is important for synaptic plasticity in learning and memory formation. However, sustaining of ISR may lead to the development of neuronal pathologies and altered patterns in behavior and perception.



Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
amyotrophic lateral sclerosis
- ATF4:
-
cAMP element binding transcription factors 4
- BiP:
-
binding immunoglobulin protein or 78-kDa glucose regulated protein
- CHOP:
-
growth arrest and DNA damage-inducible/C/EBP homologous protein (also GADD153)
- eIF:
-
translation initiation factor
- ER:
-
endoplasmic reticulum
- FTD:
-
frontotemporal dementia
- GADD34:
-
growth arrest and DNA damage-inducible protein 34
- GCN2:
-
general control nonderepressible 2 kinase
- HD:
-
Huntington’s disease
- ISR:
-
integrated stress response
- ISRIB:
-
integrated stress response inhibitor
- PD:
-
Parkinson’s disease
- PERK:
-
a PKR-like endoplasmic reticulum (ER) kinase
- PKR:
-
protein kinase R
- ROS:
-
reactive oxygen species
- SG:
-
stress granules
- TC:
-
ternary complex
- uORF:
-
upstream open reading frame
- UPR:
-
unfolded protein response
- UTR:
-
untranslated region
References
Ron, D. (2002) Translational control in the endoplasmic reticulum stress response, J Clin. Invest., 110, 1383-1388, https://doi.org/10.1172/jci16784.
Chesnokova, E., Bal, N., and Kolosov, P. (2017) Kinases of eIF2a switch translation of mRNA subset during neuronal plasticity, Int. J. Mol. Sci., 18, 2213, https://doi.org/10.3390/ijms18102213.
Costa-Mattioli, M., and Walter, P. (2020) The integrated stress response: From mechanism to disease, Science, 368, eaat5314, https://doi.org/10.1126/science.aat5314.
Shacham, T., Patel, C., and Lederkremer, G. Z. (2021) PERK pathway and neurodegenerative disease: To inhibit or to activate? Biomolecules, 11, 354, https://doi.org/10.3390/biom11030354.
Hershey, J. W. B., and Merrick, W. C. (2000) Pathway and Mechanism of Initiation of Protein Synthesis, in Translational Control of Gene Expression (Sonenberg, N., Hershey, J. W. B., and Mathews, M. B., eds.) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp. 33-88.
Jackson, R. J., Hellen, C. U., and Pestova, T. V. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation, Nat. Rev. Mol. Cell Biol., 11, 113-127, https://doi.org/10.1038/nrm2838.
Korneeva, N. L., Lamphear, B. J., Hennigan, F. L., and Rhoads, R. E. (2000) Mutually cooperative binding of eukaryotic translation initiation factor (eIF) 3 and eIF4A to human eIF4G-1, J. Biol. Chem., 275, 41369-41376, https://doi.org/10.1074/jbc.M007525200.
LeFebvre, A. K., Korneeva, N. L., Trutschl, M., Cvek, U., Duzan, R. D., et al. (2006) Translation initiation factor eIF4G-1 binds to eIF3 through the eIF3e subunit, J. Biol. Chem., 281, 22917-22932, https://doi.org/10.1074/jbc.M605418200.
Slepenkov, S. V., Korneeva, N. L., and Rhoads, R. E. (2008) Kinetic mechanism for assembly of the m7GpppG.eIF4E.eIF4G complex, J. Biol. Chem., 283, 25227-25237, https://doi.org/10.1074/jbc.M801786200.
Korneeva, N. L., First, E. A., Benoit, C. A., and Rhoads, R. E. (2005) Interaction between the NH2-terminal domain of eIF4A and the central domain of eIF4G modulates RNA-stimulated ATPase activity, J. Biol. Chem., 280, 1872-1881, https://doi.org/10.1074/jbc.M406168200.
Jennings, M. D., Zhou, Y., Mohammad-Qureshi, S. S., Bennett, D., and Pavitt, G. D. (2013) eIF2B promotes eIF5 dissociation from eIF2*GDP to facilitate guanine nucleotide exchange for translation initiation, Genes Dev., 27, 2696-2707, https://doi.org/10.1101/gad.231514.113.
Wang, X., Paulin, F. E., Campbell, L. E., Gomez, E., O’Brien, K., et al. (2001) Eukaryotic initiation factor 2B: identification of multiple phosphorylation sites in the epsilon-subunit and their functions in vivo, EMBO J., 20, 4349-4359, https://doi.org/10.1093/emboj/20.16.4349.
Starck, S. R., Tsai, J. C., Chen, K., Shodiya, M., Wang, L., et al. (2016) Translation from the 5′ untranslated region shapes the integrated stress response, Science, 351, aad3867, https://doi.org/10.1126/science.aad3867.
Calvo, S. E., Pagliarini, D. J., and Mootha, V. K. (2009) Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans, Proc. Natl. Acad. Sci. USA, 106, 7507-7512, https://doi.org/10.1073/pnas.0810916106.
Morris, D. R., and Geballe, A. P. (2000) Upstream open reading frames as regulators of mRNA translation, Mol. Cell Biol., 20, 8635-8642, https://doi.org/10.1128/mcb.20.23.8635-8642.2000.
Peccarelli, M., and Kebaara, B. W. (2014) Regulation of natural mRNAs by the nonsense-mediated mRNA decay pathway, Eukaryot. Cell, 13, 1126-1135, https://doi.org/10.1128/ec.00090-14.
Andreev, D. E., O’Connor, P. B., Fahey, C., Kenny, E. M., Terenin, I. M., et al. (2015) Translation of 5′ leaders is pervasive in genes resistant to eIF2 repression, eLife, 4, e03971, https://doi.org/10.7554/eLife.03971.
Hinnebusch, A. G. (2005) Translational regulation of GCN4 and the general amino acid control of yeast, Annu. Rev. Microbiol., 59, 407-450, https://doi.org/10.1146/annurev.micro.59.031805.133833.
Harding, H. P., Novoa, I., Zhang, Y., Zeng, H., Wek, R., et al. (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells, Mol. Cell, 6, 1099-1108, https://doi.org/10.1016/S1097-2765(00)00108-8.
Vattem, K. M., and Wek, R. C. (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells, Proc. Natl. Acad. Sci. USA, 101, 11269-11274, https://doi.org/10.1073/pnas.0400541101.
Lu, P. D., Harding, H. P., and Ron, D. (2004) Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response, J. Cell Biol., 167, 27-33, https://doi.org/10.1083/jcb.200408003.
Watatani, Y., Ichikawa, K., Nakanishi, N., Fujimoto, M., Takeda, H., et al. (2008) Stress-induced translation of ATF5 mRNA is regulated by the 5′-untranslated region, J. Biol. Chem., 283, 2543-2553, https://doi.org/10.1074/jbc.M707781200.
Dmitriev, S. E., Terenin, I. M., Andreev, D. E., Ivanov, P. A., Dunaevsky, J. E., et al. (2010) GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor, J. Biol. Chem., 285, 26779-26787, https://doi.org/10.1074/jbc.M110.119693.
Vasudevan, D., Neuman, S. D., Yang, A., Lough, L., Brown, B., et al. (2020) Translational induction of ATF4 during integrated stress response requires noncanonical initiation factors eIF2D and DENR, Nat. Commun., 11, 4677, https://doi.org/10.1038/s41467-020-18453-1.
Bohlen, J., Harbrecht, L., Blanco, S., Clemm von Hohenberg, K., Fenzl, K., et al. (2020) DENR promotes translation reinitiation via ribosome recycling to drive expression of oncogenes including ATF4, Nat. Commun., 11, 4676, https://doi.org/10.1038/s41467-020-18452-2.
Lu, P. D., Jousse, C., Marciniak, S. J., Zhang, Y., Novoa, I., et al. (2004) Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2, EMBO J., 23, 169-179, https://doi.org/10.1038/sj.emboj.7600030.
Harding, H. P., Zhang, Y., Zeng, H., Novoa, I., Lu, P. D., et al. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress, Mol. Cell, 11, 619-633, https://doi.org/10.1016/s1097-2765(03)00105-9.
Back, S. H., Scheuner, D., Han, J., Song, B., Ribick, M., et al. (2009) Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells, Cell Metab., 10, 13-26, https://doi.org/10.1016/j.cmet.2009.06.002.
Pakos-Zebrucka, K., Koryga, I., Mnich, K., Ljujic, M., Samali, A., et al. (2016) The integrated stress response, EMBO Rep., 17, 1374-1395, https://doi.org/10.15252/embr.201642195.
Fawcett, T. W., Martindale, J. L., Guyton, K. Z., Hai, T., and Holbrook, N. J. (1999) Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response, Biochem. J., 339 (Pt 1), 135-141.
Palam, L. R., Baird, T. D., and Wek, R. C. (2011) Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation, J. Biol. Chem., 286, 10939-10949, https://doi.org/10.1074/jbc.M110.216093.
Zinszner, H., Kuroda, M., Wang, X., Batchvarova, N., Lightfoot, R. T., et al. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum, Genes Dev., 12, 982-995, https://doi.org/10.1101/gad.12.7.982.
Wang, X. Z., Kuroda, M., Sok, J., Batchvarova, N., Kimmel, R., et al. (1998) Identification of novel stress-induced genes downstream of chop, EMBO J., 17, 3619-3630, https://doi.org/10.1093/emboj/17.13.3619.
Jauhiainen, A., Thomsen, C., Strombom, L., Grundevik, P., Andersson, C., et al. (2012) Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153, PLoS One, 7, e33208, https://doi.org/10.1371/journal.pone.0033208.
Brostrom, M. A., Lin, X., Cade, C., Gmitter, D., and Brostrom, C. O. (1989) Loss of a calcium requirement for protein synthesis in pituitary cells following thermal or chemical stress, J. Biol. Chem., 264, 1638-1643, https://doi.org/10.1016/S0021-9258(18)94234-1.
Novoa, I., Zhang, Y., Zeng, H., Jungreis, R., Harding, H. P., et al. (2003) Stress-induced gene expression requires programmed recovery from translational repression, EMBO J., 22, 1180-1187, https://doi.org/10.1093/emboj/cdg112.
Novoa, I., Zeng, H., Harding, H. P., and Ron, D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha, J. Cell Biol., 153, 1011-1022, https://doi.org/10.1083/jcb.153.5.1011.
Marciniak, S. J., Yun, C. Y., Oyadomari, S., Novoa, I., Zhang, Y., et al. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum, Genes Dev., 18, 3066-3077, https://doi.org/10.1101/gad.1250704.
Endo, M., Mori, M., Akira, S., and Gotoh, T. (2006) C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation, J. Immunol., 176, 6245-6253, https://doi.org/10.4049/jimmunol.176.10.6245.
Wek, R. C., Jiang, H. Y., and Anthony, T. G. (2006) Coping with stress: eIF2 kinases and translational control, Biochem. Soc. Trans., 34, 7-11, https://doi.org/10.1042/BST20060007.
Wu, C. C., Peterson, A., Zinshteyn, B., Regot, S., and Green, R. (2020) Ribosome collisions trigger general stress responses to regulate cell fate, Cell, 182, 404-416.e14, https://doi.org/10.1016/j.cell.2020.06.006.
Lavoie, H., Li, J. J., Thevakumaran, N., Therrien, M., and Sicheri, F. (2014) Dimerization-induced allostery in protein kinase regulation, Trends Biochem. Sci., 39, 475-486, https://doi.org/10.1016/j.tibs.2014.08.004.
Jousse, C., Oyadomari, S., Novoa, I., Lu, P., Zhang, Y., et al. (2003) Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells, J. Cell Biol., 163, 767-775, https://doi.org/10.1083/jcb.200308075.
Hetz, C., and Saxena, S. (2017) ER stress and the unfolded protein response in neurodegeneration, Nat. Rev. Neurol., 13, 477-491, https://doi.org/10.1038/nrneurol.2017.99.
Hamos, J. E., Oblas, B., Pulaski-Salo, D., Welch, W. J., Bole, D. G., et al. (1991) Expression of heat shock proteins in Alzheimer’s disease, Neurology, 41, 345-350, https://doi.org/10.1212/wnl.41.3.345.
Welch, W. J., and Gambetti, P. (1998) Chaperoning brain diseases, Nature, 392, 23-24, https://doi.org/10.1038/32049.
Haass, C., and Selkoe, D. J. (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide, Nat. Rev. Mol. Cell Biol., 8, 101-112, https://doi.org/10.1038/nrm2101.
Kabakov, A. E., and Gabai, V. L. (1993) Protein aggregation as primary and characteristic cell reaction to various stresses, Experientia, 49, 706-710, https://doi.org/10.1007/BF01923956.
Kouroku, Y., Fujita, E., Tanida, I., Ueno, T., Isoai, A., et al. (2007) ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation, Cell Death Differ., 14, 230-239, https://doi.org/10.1038/sj.cdd.4401984.
Rzymski, T., Milani, M., Pike, L., Buffa, F., Mellor, H. R., et al. (2010) Regulation of autophagy by ATF4 in response to severe hypoxia, Oncogene, 29, 4424-4435, https://doi.org/10.1038/onc.2010.191.
Nemoto, N., Udagawa, T., Ohira, T., Jiang, L., Hirota, K., et al. (2010) The roles of stress-activated Sty1 and Gcn2 kinases and of the protooncoprotein homologue Int6/eIF3e in responses to endogenous oxidative stress during histidine starvation, J. Mol. Biol., 404, 183-201, https://doi.org/10.1016/j.jmb.2010.09.016.
Zhan, K., Vattem, K. M., Bauer, B. N., Dever, T. E., Chen, J. J., et al. (2002) Phosphorylation of eukaryotic initiation factor 2 by heme-regulated inhibitor kinase-related protein kinases in Schizosaccharomyces pombe is important for fesistance to environmental stresses, Mol. Cell. Biol., 22, 7134-7146, https://doi.org/10.1128/mcb.22.20.7134-7146.2002.
Feissner, R. F., Skalska, J., Gaum, W. E., and Sheu, S. S. (2009) Crosstalk signaling between mitochondrial Ca2+ and ROS, Front. Biosci. (Landmark Ed), 14, 1197-1218, https://doi.org/10.2741/3303.
Wolozin, B. (2012) Regulated protein aggregation: Stress granules and neurodegeneration, Mol. Neurodegener., 7, 56, https://doi.org/10.1186/1750-1326-7-56.
Buxbaum, A. R., Haimovich, G., and Singer, R. H. (2015) In the right place at the right time: visualizing and understanding mRNA localization, Nat. Rev. Mol. Cell Biol., 16, 95-109, https://doi.org/10.1038/nrm3918.
Terenzio, M., Schiavo, G., and Fainzilber, M. (2017) Compartmentalized signaling in neurons: From cell biology to neuroscience, Neuron, 96, 667-679, https://doi.org/10.1016/j.neuron.2017.10.015.
Anderson, P., and Kedersha, N. (2008) Stress granules: The Tao of RNA triage, Trends Biochem. Sci., 33, 141-150, https://doi.org/10.1016/j.tibs.2007.12.003.
Kiebler, M. A., and Bassell, G. J. (2006) Neuronal RNA granules: movers and makers, Neuron, 51, 685-690, https://doi.org/10.1016/j.neuron.2006.08.021.
Jain, S., and Parker, R. (2013) The discovery and analysis of P Bodies, Adv. Exp. Med. Biol., 768, 23-43, https://doi.org/10.1007/978-1-4614-5107-5_3.
Buchan, J. R. (2014) mRNP granules. Assembly, function, and connections with disease, RNA Biol., 11, 1019-1030, https://doi.org/10.4161/15476286.2014.972208.
Liu-Yesucevitz, L., Bilgutay, A., Zhang, Y. J., Vanderweyde, T., Citro, A., et al. (2010) Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue, PLoS One, 5, e13250, https://doi.org/10.1371/journal.pone.0013250.
Thomas, M. G., Loschi, M., Desbats, M. A., and Boccaccio, G. L. (2011) RNA granules: the good, the bad and the ugly, Cell. Signall., 23, 324-334, https://doi.org/10.1016/j.cellsig.2010.08.011.
Vanderweyde, T., Yu, H., Varnum, M., Liu-Yesucevitz, L., Citro, A., et al. (2012) Contrasting pathology of the stress granule proteins TIA-1 and G3BP in tauopathies, J. Neurosci., 32, 8270-8283, https://doi.org/10.1523/jneurosci.1592-12.2012.
Vanderweyde, T., Apicco, D. J., Youmans-Kidder, K., Ash, P. E., Cook, C., et al. (2016) Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity, Cell Rep., 15, 1455-1466, https://doi.org/10.1016/j.celrep.2016.04.045.
Bentmann, E., Haass, C., and Dormann, D. (2013) Stress granules in neurodegeneration – lessons learnt from TAR DNA binding protein of 43 kDa and fused in sarcoma, FEBS J., 280, 4348-4370, https://doi.org/10.1111/febs.12287.
Abrakhi, S., Kretov, D. A., Desforges, B., Dobra, I., Bouhss, A., et al. (2017) Nanoscale analysis reveals the maturation of neurodegeneration-associated protein aggregates: Grown in mRNA granules then released by stress granule proteins, ACS Nano, 11, 7189-7200, https://doi.org/10.1021/acsnano.7b03071.
Han, J., Back, S. H., Hur, J., Lin, Y. H., Gildersleeve, R., et al. (2013) ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death, Nat. Cell Biol., 15, 481-490, https://doi.org/10.1038/ncb2738.
Galehdar, Z., Swan, P., Fuerth, B., Callaghan, S. M., Park, D. S., et al. (2010) Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4-CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA, J. Neurosci., 30, 16938-16948, https://doi.org/10.1523/jneurosci.1598-10.2010.
Ren, D., Tu, H. C., Kim, H., Wang, G. X., Bean, G. R., et al. (2010) BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program, Science, 330, 1390-1393, https://doi.org/10.1126/science.1190217.
Paschen, W., Gissel, C., Linden, T., Althausen, S., and Doutheil, J. (1998) Activation of gadd153 expression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum dysfunction, Brain Res. Mol. Brain Res., 60, 115-122, https://doi.org/10.1016/s0169-328x(98)00180-6.
Jin, K., Mao, X. O., Eshoo, M. W., Nagayama, T., Minami, M., et al. (2001) Microarray analysis of hippocampal gene expression in global cerebral ischemia, Ann. Neurol., 50, 93-103, https://doi.org/10.1002/ana.1073.
Morimoto, N., Oida, Y., Shimazawa, M., Miura, M., Kudo, T., et al. (2007) Involvement of endoplasmic reticulum stress after middle cerebral artery occlusion in mice, Neuroscience, 147, 957-967, https://doi.org/10.1016/j.neuroscience.2007.04.017.
Niizuma, K., Endo, H., Nito, C., Myer, D. J., and Chan, P. H. (2009) Potential role of PUMA in delayed death of hippocampal CA1 neurons after transient global cerebral ischemia, Stroke, 40, 618-625, https://doi.org/10.1161/strokeaha.108.524447.
Holtz, W. A., and O’Malley, K. L. (2003) Parkinsonian mimetics induce aspects of unfolded protein response in death of dopaminergic neurons, J. Biol. Chem., 278, 19367-19377, https://doi.org/10.1074/jbc.M211821200.
Silva, R. M., Ries, V., Oo, T. F., Yarygina, O., Jackson-Lewis, V., et al. (2005) CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism, J. Neurochem., 95, 974-986, https://doi.org/10.1111/j.1471-4159.2005.03428.x.
Kieran, D., Woods, I., Villunger, A., Strasser, A., and Prehn, J. H. (2007) Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice, Proc. Natl. Acad. Sci. USA, 104, 20606-20611, https://doi.org/10.1073/pnas.0707906105.
Hiramatsu, N., Messah, C., Han, J., LaVail, M. M., Kaufman, R. J., et al. (2014) Translational and posttranslational regulation of XIAP by eIF2alpha and ATF4 promotes ER stress-induced cell death during the unfolded protein response, Mol. Biol. Cell, 25, 1411-1420, https://doi.org/10.1091/mbc.E13-11-0664.
Chang, R. C., Wong, A. K., Ng, H. K., and Hugon, J. (2002) Phosphorylation of eukaryotic initiation factor-2alpha (eIF2alpha) is associated with neuronal degeneration in Alzheimer’s disease, Neuroreport, 13, 2429-2432.
Hoozemans, J. J., van Haastert, E. S., Eikelenboom, P., de Vos, R. A., Rozemuller, J. M., et al. (2007) Activation of the unfolded protein response in Parkinson’s disease, Biochem. Biophys. Res. Commun., 354, 707-711, https://doi.org/10.1016/j.bbrc.2007.01.043.
Atkin, J. D., Farg, M. A., Walker, A. K., McLean, C., Tomas, D., et al. (2008) Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis, Neurobiol. Dis., 30, 400-407, https://doi.org/10.1016/j.nbd.2008.02.009.
Chou, A., Krukowski, K., Jopson, T., Zhu, P. J., Costa-Mattioli, M., et al. (2017) Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury, Proc. Natl. Acad. Sci. USA, 114, E6420-E6426, https://doi.org/10.1073/pnas.1707661114.
Nijholt, D. A., van Haastert, E. S., Rozemuller, A. J., Scheper, W., and Hoozemans, J. J. (2012) The unfolded protein response is associated with early tau pathology in the hippocampus of tauopathies, J. Pathol., 226, 693-702, https://doi.org/10.1002/path.3969.
Fernández-Vizarra, P., Fernández, A. P., Castro-Blanco, S., Serrano, J., Bentura, M. L., et al. (2004) Intra- and extracellular Abeta and PHF in clinically evaluated cases of Alzheimer’s disease, Histol. Histopathol., 19, 823-844, https://doi.org/10.14670/hh-19.823.
O'Connor, T., Sadleir, K. R., Maus, E., Velliquette, R. A., Zhao, J., et al. (2008) Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis, Neuron, 60, 988-1009, https://doi.org/10.1016/j.neuron.2008.10.047.
Mouton-Liger, F., Paquet, C., Dumurgier, J., Bouras, C., Pradier, L., et al. (2012) Oxidative stress increases BACE1 protein levels through activation of the PKR-eIF2alpha pathway, Biochim. Biophys. Acta, 1822, 885-896, https://doi.org/10.1016/j.bbadis.2012.01.009.
Ma, T., Trinh, M. A., Wexler, A. J., Bourbon, C., Gatti, E., et al. (2013) Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits, Nat. Neurosci., 16, 1299-1305, https://doi.org/10.1038/nn.3486.
Baleriola, J., Walker, C. A., Jean, Y. Y., Crary, J. F., Troy, C. M., et al. (2014) Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions, Cell, 158, 1159-1172, https://doi.org/10.1016/j.cell.2014.07.001.
Lange, P. S., Chavez, J. C., Pinto, J. T., Coppola, G., Sun, C. W., et al. (2008) ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo, J. Exp. Med., 205, 1227-1242, https://doi.org/10.1084/jem.20071460.
Yang, W., Zhou, X., Zimmermann, H. R., Cavener, D. R., Klann, E., et al. (2016) Repression of the eIF2α kinase PERK alleviates mGluR-LTD impairments in a mouse model of Alzheimer’s disease, Neurobiol. Aging, 41, 19-24, https://doi.org/10.1016/j.neurobiolaging.2016.02.005.
Gully, J. C., Sergeyev, V. G., Bhootada, Y., Mendez-Gomez, H., Meyers, C. A., et al. (2016) Up-regulation of activating transcription factor 4 induces severe loss of dopamine nigral neurons in a rat model of Parkinson’s disease, Neurosci. Lett., 627, 36-41, https://doi.org/10.1016/j.neulet.2016.05.039.
Bellucci, A., Navarria, L., Zaltieri, M., Falarti, E., Bodei, S., et al. (2011) Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson’s disease, J. Neurochem., 116, 588-605, https://doi.org/10.1111/j.1471-4159.2010.07143.x.
Cooper, A. A., Gitler, A. D., Cashikar, A., Haynes, C. M., Hill, K. J., et al. (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models, Science, 313, 324-328, https://doi.org/10.1126/science.1129462.
Ilieva, E. V., Ayala, V., Jové, M., Dalfó, E., Cacabelos, D., et al. (2007) Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis, Brain, 130, 3111-3123, https://doi.org/10.1093/brain/awm190.
Kikuchi, H., Almer, G., Yamashita, S., Guégan, C., Nagai, M., et al. (2006) Spinal cord endoplasmic reticulum stress associated with a microsomal accumulation of mutant superoxide dismutase-1 in an ALS model, Proc. Natl. Acad. Sci. USA, 103, 6025-6030, https://doi.org/10.1073/pnas.0509227103.
Urushitani, M., Ezzi, S. A., Matsuo, A., Tooyama, I., and Julien, J. P. (2008) The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS, FASEB J., 22, 2476-2487, https://doi.org/10.1096/fj.07-092783.
Farg, M. A., Soo, K. Y., Warraich, S. T., Sundaramoorthy, V., Blair, I. P., et al. (2013) Ataxin-2 interacts with FUS and intermediate-length polyglutamine expansions enhance FUS-related pathology in amyotrophic lateral sclerosis, Human Mol. Genet., 22, 717-728, https://doi.org/10.1093/hmg/dds479.
Hetz, C., Russelakis-Carneiro, M., Maundrell, K., Castilla, J., and Soto, C. (2003) Caspase-12 and endoplasmic reticulum stress mediate neurotoxicity of pathological prion protein, EMBO J., 22, 5435-5445, https://doi.org/10.1093/emboj/cdg537.
Carnemolla, A., Fossale, E., Agostoni, E., Michelazzi, S., Calligaris, R., et al. (2009) Rrs1 is involved in endoplasmic reticulum stress response in Huntington’s disease, J. Biol. Chem., 284, 18167-18173, https://doi.org/10.1074/jbc.M109.018325.
Vitet, H., Brandt, V., and Saudou, F. (2020) Traffic signaling: New functions of huntingtin and axonal transport in neurological disease, Curr. Opin. Neurobiol., 63, 122-130, https://doi.org/10.1016/j.conb.2020.04.001.
Kandel, E. R. (2001) The molecular biology of memory storage: a dialogue between genes and synapses, Science, 294, 1030-1038, https://doi.org/10.1126/science.1067020.
Klann, E., and Sweatt, J. D. (2008) Altered protein synthesis is a trigger for long-term memory formation, Neurobiol. Learn Mem., 89, 247-259, https://doi.org/10.1016/j.nlm.2007.08.009.
Trinh, M. A., and Klann, E. (2013) Translational control by eIF2α kinases in long-lasting synaptic plasticity and long-term memory, Neurobiol. Learn Mem., 105, 93-99, https://doi.org/10.1016/j.nlm.2013.04.013.
Di Prisco, G. V., Huang, W., Buffington, S. A., Hsu, C. C., Bonnen, P. E., et al. (2014) Translational control of mGluR-dependent long-term depression and object-place learning by eIF2alpha, Nat. Neurosci., 17, 1073-1082, https://doi.org/10.1038/nn.3754.
Costa-Mattioli, M., Gobert, D., Stern, E., Gamache, K., Colina, R., et al. (2007) eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory, Cell, 129, 195-206, https://doi.org/10.1016/j.cell.2007.01.050.
Jiang, Z., Belforte, J. E., Lu, Y., Yabe, Y., Pickel, J., et al. (2010) eIF2{alpha} phosphorylation-dependent translation in CA1 pyramidal cells impairs hippocampal memory consolidation without affecting general translation, J. Neurosci., 30, 2582-2594, https://doi.org/10.1523/jneurosci.3971-09.2010.
Trinh, M. A., Ma, T., Kaphzan, H., Bhattacharya, A., Antion, M. D., et al. (2014) The eIF2α kinase PERK limits the expression of hippocampal metabotropic glutamate receptor-dependent long-term depression, Learn. Memory, 21, 298-304, https://doi.org/10.1101/lm.032219.113.
Silva, A. J., Kogan, J. H., Frankland, P. W., and Kida, S. (1998) CREB and memory, Annu. Rev. Neurosci., 21, 127-148, https://doi.org/10.1146/annurev.neuro.21.1.127.
Yin, J. C., Wallach, J. S., Del Vecchio, M., Wilder, E. L., Zhou, H., et al. (1994) Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila, Cell, 79, 49-58, https://doi.org/10.1016/0092-8674(94)90399-9.
Bartsch, D., Ghirardi, M., Skehel, P. A., Karl, K. A., Herder, S. P., et al. (1995) Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change, Cell, 83, 979-992, https://doi.org/10.1016/0092-8674(95)90213-9.
Chen, A., Muzzio, I. A., Malleret, G., Bartsch, D., Verbitsky, M., et al. (2003) Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of ATF4 (CREB-2) and C/EBP proteins, Neuron, 39, 655-669, https://doi.org/10.1016/s0896-6273(03)00501-4.
Huang, W., Placzek, A. N., Di Prisco, G. V., Khatiwada, S., Sidrauski, C., et al. (2016) Translational control by eIF2α phosphorylation regulates vulnerability to the synaptic and behavioral effects of cocaine, eLife, 5, e12052, https://doi.org/10.7554/eLife.12052.
Nadif Kasri, N., Nakano-Kobayashi, A., and Van Aelst, L. (2011) Rapid synthesis of the X-linked mental retardation protein OPHN1 mediates mGluR-dependent LTD through interaction with the endocytic machinery, Neuron, 72, 300-315, https://doi.org/10.1016/j.neuron.2011.09.001.
Costa-Mattioli, M., Gobert, D., Harding, H., Herdy, B., Azzi, M., et al. (2005) Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2, Nature, 436, 1166-1173, https://doi.org/10.1038/nature03897.
Zhu, P. J., Huang, W., Kalikulov, D., Yoo, J. W., Placzek, A. N., et al. (2011) Suppression of PKR promotes network excitability and enhanced cognition by interferon-γ-mediated disinhibition, Cell, 147, 1384-1396, https://doi.org/10.1016/j.cell.2011.11.029.
Trinh, M. A., Kaphzan, H., Wek, R. C., Pierre, P., Cavener, D. R., et al. (2012) Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility, Cell Rep., 1, 676-688, https://doi.org/10.1016/j.celrep.2012.04.010.
Liu, J., Pasini, S., Shelanski, M. L., and Greene, L. A. (2014) Activating transcription factor 4 (ATF4) modulates post-synaptic development and dendritic spine morphology, Front. Cell. Neurosci., 8, 177, https://doi.org/10.3389/fncel.2014.00177.
Moreno, J. A., Radford, H., Peretti, D., Steinert, J. R., Verity, N., et al. (2012) Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration, Nature, 485, 507-511, https://doi.org/10.1038/nature11058.
Green, T. A., Alibhai, I. N., Unterberg, S., Neve, R. L., Ghose, S., et al. (2008) Induction of activating transcription factors (ATFs) ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior, J. Neurosci., 28, 2025-2032, https://doi.org/10.1523/jneurosci.5273-07.2008.
Fan, R., Schrott, L. M., Snelling, S., Ndi, J., Arnold, T.,et al. (2015) Chronic oxycodone induces integrated stress response in rat brain, BMC Neurosci., 16, 58, https://doi.org/10.1186/s12868-015-0197-8.
Fan, R., Schrott, L. M., Arnold, T., Snelling, S., Rao, M., et al. (2018) Chronic oxycodone induces axonal degeneration in rat brain, BMC Neurosci., 19, 15, https://doi.org/10.1186/s12868-018-0417-0.
Fan, R., Schrott, L. M., Snelling, S., Felty, J., Graham, D., et al. (2020) Carbonyl-protein content increases in brain and blood of female rats after chronic oxycodone treatment, BMC Neurosci., 21, 4, https://doi.org/10.1186/s12868-020-0552-2.
Ballesteros-Yanez, I., Ambrosio, E., Benavides-Piccione, R., Perez, J., et al. (2007) The effects of morphine self-administration on cortical pyramidal cell structure in addiction-prone lewis rats, Cerebral Cortex, 17, 238-249, https://doi.org/10.1093/cercor/bhj142.
Placzek, A. N., Di Prisco, G. V., Khatiwada, S., Sgritta, M., Huang, W., et al. (2016) eIF2α-mediated translational control regulates the persistence of cocaine-induced LTP in midbrain dopamine neurons, eLife, 5, e17517, https://doi.org/10.7554/eLife.17517.
Placzek, A. N., Molfese, D. L., Khatiwada, S., Di Prisco, G. V., Huang, W., et al. (2016) Translational control of nicotine-evoked synaptic potentiation in mice and neuronal responses in human smokers by eIF2α, eLife, 5, e12056, https://doi.org/10.7554/eLife.12056.
Melas, P. A., Qvist, J. S., Deidda, M., Upreti, C., Wei, Y. B., et al. (2018) Cannabinoid modulation of eukaryotic initiation factors (eIF2α and eIF2B1) and behavioral cross-sensitization to cocaine in adolescent rats, Cell Rep., 22, 2909-2923, https://doi.org/10.1016/j.celrep.2018.02.065.
Price, T. J., and Géranton, S. M. (2009) Translating nociceptor sensitivity: the role of axonal protein synthesis in nociceptor physiology, Eur. J. Neurosci., 29, 2253-2263, https://doi.org/10.1111/j.1460-9568.2009.06786.x.
Melemedjian, O. K., and Khoutorsky, A. (2015) Translational control of chronic pain, Prog. Mol. Biol. Transl. Sci., 131, 185-213, https://doi.org/10.1016/bs.pmbts.2014.11.006.
Khoutorsky, A., and Price, T. J. (2018) Translational control mechanisms in persistent pain, Trends Neurosci., 41, 100-114, https://doi.org/10.1016/j.tins.2017.11.006.
Yousuf, M. S., Shiers, S. I., Sahn, J. J., and Price, T. J. (2021) Pharmacological manipulation of translation as a therapeutic target for chronic pain, Pharmacol. Rev., 73, 59-88, https://doi.org/10.1124/pharmrev.120.000030.
Géranton, S. M., Jiménez-Díaz, L., Torsney, C., Tochiki, K. K., Stuart, S. A., et al. (2009) A rapamycin-sensitive signaling pathway is essential for the full expression of persistent pain states, J. Neurosci., 29, 15017-15027, https://doi.org/10.1523/jneurosci.3451-09.2009.
Asante, C. O., Wallace, V. C., and Dickenson, A. H. (2010) Mammalian target of rapamycin signaling in the spinal cord is required for neuronal plasticity and behavioral hypersensitivity associated with neuropathy in the rat, J. Pain Official J. Am. Pain Soc., 11, 1356-1367, https://doi.org/10.1016/j.jpain.2010.03.013.
Obara, I., Tochiki, K. K., Géranton, S. M., Carr, F. B., Lumb, B. M., et al. (2011) Systemic inhibition of the mammalian target of rapamycin (mTOR) pathway reduces neuropathic pain in mice, Pain, 152, 2582-2595, https://doi.org/10.1016/j.pain.2011.07.025.
Khoutorsky, A., Sorge, R. E., Prager-Khoutorsky, M., Pawlowski, S. A., Longo, G., et al. (2016) eIF2alpha phosphorylation controls thermal nociception, Proc. Natl. Acad. Sci. USA, 113, 11949-11954, https://doi.org/10.1073/pnas.1614047113.
Braz, J. M., and Basbaum, A. I. (2010) Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli, Pain, 150, 290-301, https://doi.org/10.1016/j.pain.2010.05.005.
Dong, L., Guarino, B. B., Jordan-Sciutto, K. L., and Winkelstein, B. A. (2011) Activating transcription factor 4, a mediator of the integrated stress response, is increased in the dorsal root ganglia following painful facet joint distraction, Neuroscience, 193, 377-386, https://doi.org/10.1016/j.neuroscience.2011.07.059.
Leegwater, P. A., Vermeulen, G., Könst, A. A., Naidu, S., Mulders, J., et al. (2001) Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter, Nat. Genet., 29, 383-388, https://doi.org/10.1038/ng764.
Van der Knaap, M. S., Leegwater, P. A., Konst, A. A., Visser, A., Naidu, S., et al. (2002) Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter, Ann. Neurol., 51, 264-270, https://doi.org/10.1002/ana.10112.
Bugiani, M., Boor, I., Powers, J. M., Scheper, G. C., and van der Knaap, M. S. (2010) Leukoencephalopathy with vanishing white matter: a review, J. Neuropathol. Exp. Neurol., 69, 987-996, https://doi.org/10.1097/NEN.0b013e3181f2eafa.
Liu, R., van der Lei, H. D., Wang, X., Wortham, N. C., Tang, H., et al. (2011) Severity of vanishing white matter disease does not correlate with deficits in eIF2B activity or the integrity of eIF2B complexes, Hum. Mutat., 32, 1036-1045, https://doi.org/10.1002/humu.21535.
Wong, Y. L., LeBon, L., Basso, A. M., Kohlhaas, K. L., Nikkel, A. L., et al. (2019) eIF2B activator prevents neurological defects caused by a chronic integrated stress response, eLife, 8, e42940, https://doi.org/10.7554/eLife.42940.
Wong, Y. L., LeBon, L., Edalji, R., Lim, H. B., Sun, C., et al. (2018) The small molecule ISRIB rescues the stability and activity of Vanishing White Matter Disease eIF2B mutant complexes, eLife, 7, e32733, https://doi.org/10.7554/eLife.32733.
Borck, G., Shin, B. S., Stiller, B., Mimouni-Bloch, A., Thiele, H., et al. (2012) eIF2gamma mutation that disrupts eIF2 complex integrity links intellectual disability to impaired translation initiation, Mol. Cell, 48, 641-646, https://doi.org/10.1016/j.molcel.2012.09.005.
Julier, C., and Nicolino, M. (2010) Wolcott–Rallison syndrome, Orphanet J. Rare Dis., 5, 29, https://doi.org/10.1186/1750-1172-5-29.
Eyries, M., Montani, D., Girerd, B., Perret, C., Leroy, A., et al. (2014) EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension, Nat. Genet., 46, 65-69, https://doi.org/10.1038/ng.2844.
Longchamp, A., Mirabella, T., Arduini, A., MacArthur, M. R., Das, A., et al. (2018) Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H(2)S production, Cell, 173, 117-129.e114, https://doi.org/10.1016/j.cell.2018.03.001.
Rubovitch, V., Barak, S., Rachmany, L., Goldstein, R. B., Zilberstein, Y., et al. (2015) The neuroprotective effect of salubrinal in a mouse model of traumatic brain injury, Neuromol. Med., 17, 58-70, https://doi.org/10.1007/s12017-015-8340-3.
Halliday, M., Radford, H., Zents, K. A. M., Molloy, C., Moreno, J. A., et al. (2017) Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice, Brain, 140, 1768-1783, https://doi.org/10.1093/brain/awx074.
Moreno, J. A., Halliday, M., Molloy, C., Radford, H., Verity, N., et al. (2013) Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice, Sci. Transl. Med., 5, 206ra138, https://doi.org/10.1126/scitranslmed.3006767.
Radford, H., Moreno, J. A., Verity, N., Halliday, M., and Mallucci, G. R. (2015) PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia, Acta Neuropathol., 130, 633-642, https://doi.org/10.1007/s00401-015-1487-z.
Rojas-Rivera, D., Delvaeye, T., Roelandt, R., Nerinckx, W., Augustyns, K., et al. (2017) When PERK inhibitors turn out to be new potent RIPK1 inhibitors: critical issues on the specificity and use of GSK2606414 and GSK2656157, Cell Death Differ., 24, 1100-1110, https://doi.org/10.1038/cdd.2017.58.
Halliday, M., Radford, H., Sekine, Y., Moreno, J., Verity, N., et al. (2015) Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity, Cell Death Dis., 6, e1672, https://doi.org/10.1038/cddis.2015.49.
Bruch, J., Xu, H., Rösler, T. W., De Andrade, A., Kuhn, P. H., et al. (2017) PERK activation mitigates tau pathology in vitro and in vivo, EMBO Mol. Med., 9, 371-384, https://doi.org/10.15252/emmm.201606664.
Sidrauski, C., Acosta-Alvear, D., Khoutorsky, A., Vedantham, P., Hearn, B. R., et al. (2013) Pharmacological brake-release of mRNA translation enhances cognitive memory, eLife Sci., 2, e00498, https://doi.org/10.7554/eLife.00498.
Sidrauski, C., Tsai, J. C., Kampmann, M., Hearn, B. R., Vedantham, P., et al. (2015) Pharmacological dimerization and activation of the exchange factor eIF2B antagonizes the integrated stress response, eLife, 4, e07314, https://doi.org/10.7554/eLife.07314.
Sekine, Y., Zyryanova, A., Crespillo-Casado, A., Fischer, P. M., Harding, H. P., et al. (2015) Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound, Science, 348, 1027-1030, https://doi.org/10.1126/science.aaa6986.
Tsai, J. C., Miller-Vedam, L. E., Anand, A. A., Jaishankar, P., Nguyen, H. C., et al. (2018) Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule, Science, 359, eaaq0939, https://doi.org/10.1126/science.aaq0939.
Zyryanova, A. F., Weis, F., Faille, A., Alard, A. A., Crespillo-Casado, A., et al. (2018) Binding of ISRIB reveals a regulatory site in the nucleotide exchange factor eIF2B, Science, 359, 1533-1536, https://doi.org/10.1126/science.aar5129.
Rabouw, H. H., Langereis, M. A., Anand, A. A., Visser, L. J., de Groot, R. J., et al. (2019) Small molecule ISRIB suppresses the integrated stress response within a defined window of activation, Proc. Natl. Acad. Sci. USA, 116, 2097-2102, https://doi.org/10.1073/pnas.1815767116.
Hu, J., Rho, H. S., Newman, R. H., Hwang, W., Neiswinger, J., et al. (2014) Global analysis of phosphorylation networks in humans, Biochim. Biophys. Acta, 1844, 224-231, https://doi.org/10.1016/j.bbapap.2013.03.009.
Acknowledgments
The author is grateful to the Department of Pharmacology, Toxicology and Neuroscience, the Department of Emergency Medicine, and Feist-Weiller Cancer Center for their support. The author expresses her gratitude to Dr. Dmitry N. Lyabin and Evgeniya V. Serebrova for help in manuscript preparation.
Funding
This study was financially supported by NIH (Grants Nos. 2R01GM20818, R01GM20818, and R01GM20818-32).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares no conflicts of interest. This article does not contain any studies with human participants or animals performed by the author.
Additional information
Translated from Uspekhi Biologicheskoi Khimii, 2022, Vol. 62, pp. 243-278.
In memory of a great scientist and my Teacher
and Mentor Lev Pavlovich Ovchinnikov
Rights and permissions
About this article
Cite this article
Korneeva, N.L. Integrated Stress Response in Neuronal Pathology and in Health. Biochemistry Moscow 87 (Suppl 1), S111–S127 (2022). https://doi.org/10.1134/S0006297922140103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297922140103