Abstract
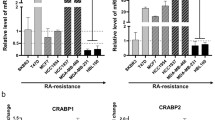
Retinoic acid (RA) binding proteins, CRABP1 and CRABP2, are molecular chaperones that mediate intracellular activity of RA, the key promoter of cell differentiation with tumor suppressor activity. One of the main functions of CRABP2 is delivery and transfer of RA to the nuclear receptors RAR/RXR, which leads to activation of the transcription of a wide range of retinoid-responsive genes. The functions of CRABP1 are less studied but are apparently associated with sequestration of RA in cytoplasm and limitation of its transcriptional activity, suggesting involvement of this protein in the development of RA resistance. The mechanisms regulating activity of CRABP1 are also poorly understood. Comparison of the CRABP1 level in tumor cell lines of various origins, performed for the first time here, showed absence of the CRABP1 protein in the cell lines of tumors considered to be RA-resistant, and pronounced production of this protein in the RA-sensitive cells. However, analysis carried out with a panel of breast cancer cell lines with different levels of RA-sensitivity showed that there was no correlation between the production of CRABP1 protein and the sensitivity of the cells to RA. At the same time, we found strong correlation between the expression of CRABP1 and CRABP2 proteins in all studied cell types, regardless of their origin and RA-sensitivity/resistance. Moreover, suppression of the CRABP1 level in both RA-sensitive and RA-resistant cells was shown in the cells with cells with knockdown of CRABP2 gene. The revealed CRABP2-dependent regulation of CRABP1 production is a new mechanism of the intracellular retinoic signaling system.





Similar content being viewed by others
Abbreviations
- ATRA:
-
all-trans retinoic acid
- BC:
-
breast cancer
- CRABP1 and CRABP2:
-
cellular retinoic acid binding proteins 1 and 2
- FABP5:
-
fatty acid binding protein 5
- GFP:
-
green fluorescent protein
- NSCLC:
-
non-small cell lung cancer
- PPAR:
-
peroxisome proliferator-activated receptors
- RA:
-
retinoic acid
- RAR:
-
retinoic acid receptor
References
Connolly, R. M., Nguyen, N. K., and Sukumar, S. (2013) Molecular pathways: current role and future directions of the retinoic acid pathway in cancer prevention and treatment, Clin. Cancer Res., 19, 1651-1959, https://doi.org/10.1158/1078-0432.CCR-12-3175.
Schenk, T., Stengel, S., and Zelent, A. (2014) Unlocking the potential of retinoic acid in anticancer therapy, Br. J. Cancer, 111, 2039-2045, https://doi.org/10.1038/bjc.2014.412.
Chevkina, E. M., and Favorskaya, I. A. (2015) CRABP proteins – relatives or namesakers? [in Russian], Uspekhi Mol. Onkol., 2, 6-16, https://doi.org/10.17650/2313-805X.2015.2.2.6-16.
Tchevkina, E. M. (2017) Retinoic acid binding proteins and cancer: similarity or polarity? Cancer Ther. Oncol. Int. J., 8, 555733, https://doi.org/10.19080/ctoij.2017.08.555733.
Sussman, F., and De Lera, A. R. (2005) Ligand recognition by RAR and RXR receptors: binding and selectivity, J. Med. Chem., 48, 6212-6219, https://doi.org/10.1021/jm050285w.
Schug, T. T., Berry, D. C., Shaw, N. S., Travis, S. N., and Noy, N. (2007) Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors, Cell, 129, 723-733, https://doi.org/10.1016/j.cell.2007.02.050.
Schug, T. T., Berry, D. C., Toshkov, I. A., Cheng, L., Nikitin, A. Y., and Noy, N. (2008) Overcoming retinoic acid-resistance of mammary carcinomas by diverting retinoic acid from PPARβ/δ to RAR, Proc. Natl. Acad. Sci. USA, 105, 7546-7551, https://doi.org/10.1073/pnas.0709981105.
Liu, R. Z., Graham, K., Glubrecht, D. D., Germain, D. R., Mackey, J. R., and Godbout, R. (2011) Association of FABP5 expression with poor survival in triple-negative breast cancer: implication for retinoic acid therapy, Am. J. Pathol., 178, 997-1008, https://doi.org/10.1016/j.ajpath.2010.11.075.
Vreeland, A. C., Levi, L., Zhang, W., Berry, D. C., and Noy, N. (2014) Cellular retinoic acid-binding protein 2 inhibits tumor growth by two distinct mechanisms, J. Biol. Chem., 289, 34065-34073, https://doi.org/10.1074/jbc.M114.604041.
Vreeland, A. C., Yu, S., Levi, L., de Barros Rossetto, D., and Noy, N. (2014) Transcript stabilization by the RNA-binding protein HuR is regulated by cellular retinoic acid-binding protein 2, Mol. Cell. Biol., 34, 2135-2146, https://doi.org/10.1128/mcb.00281-14.
Mallikarjuna, K., Sundaram, C. S., Sharma, Y., Deepa, P. R., Khetan, V., et al. (2010) Comparative proteomic analysis of differentially expressed proteins in primary retinoblastoma tumors, Proteom. Clin. Appl., 4, 449-463, https://doi.org/10.1002/prca.200900069.
Liu, R. Z., Li, S., Garcia, E., Glubrecht, D. D., Yin Poon, H., et al. (2016) Association between cytoplasmic CRABP2, altered retinoic acid signaling, and poor prognosis in glioblastoma, Glia, 64, 963-976, https://doi.org/10.1002/glia.22976.
Dong, D., Ruuska, S. E., Levinthal, D. J., and Noy, N. (1999) Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid, J. Biol. Chem., 274, 23695-23698, https://doi.org/10.1074/jbc.274.34.23695.
Blaese, M. A., Santo-Hoeltje, L., and Rodemann, H. P. (2003) CRABP I expression and the mediation of the sensitivity of human tumour cells to retinoic acid and irradiation, Int. J. Radiat. Biol., 79, 981-991, https://doi.org/10.1080/09553000310001632949.
Liu, R. Z., Garcia, E., Glubrecht, D. D., Poon, H. Y., Mackey, J. R., and Godbout, R. (2015) CRABP1 is associated with a poor prognosis in breast cancer: adding to the complexity of breast cancer cell response to retinoic acid, Mol. Cancer, 14, 129, https://doi.org/10.1186/s12943-015-0380-7.
Fiorella, P. D., and Napoli, J. L. (1991) Expression of cellular retinoic acid binding protein (CRABP) in Escherichia coli. Characterization and evidence that holo-CRABP is a substrate in retinoic acid metabolism, J. Biol. Chem., 266, 16572-16579.
Boylan, J. F., and Gudas, L. J. (1992) The level of CRABP-I expression influences the amounts and types of all- trans-retinoic acid metabolites in F9 teratocarcinoma stem cells, J. Biol. Chem., 267, 21486-21491.
Won, J. Y., Nam, E. C., Yoo, S. J., Kwon, H. J., Um, S. J., et al. (2004) The effect of cellular retinoic acid binding protein-I expression on the CYP26-mediated catabolism of all-trans retinoic acid and cell proliferation in head and neck squamous cell carcinoma, Metab. Clin. Exp., 53, 1007-1012, https://doi.org/10.1016/j.metabol.2003.12.015.
Delektorskaya, V. V., Komel’kov, A. V., Zborovskaya, I. B., Enikeev, A. D., Safronova, V. M., and Chevkina, E. M. (2017) Nuclear localization of cellular retinoic acid-binding protein 1 (Crabp1) is associated with malignancy level in lung neuroendocrine tumors [in Russian], Voprosy Onkologii, 63, 886-893.
Gaub, M. P., Lutz, Y., Ghyselinck, N. B., Scheuer, I., Pfister, V., et al. (1998) Nuclear detection of cellular retinoic acid binding proteins I and II with new antibodies, J. Histochem. Cytochem., 46, 1103-1111, https://doi.org/10.1177/002215549804601002.
Favorskaya, I., Kainov, Y., Chemeris, G., Komelkov, A., Zborovskaya, I., and Tchevkina, E. (2014) Expression and clinical significance of CRABP1 and CRABP2 in non-small cell lung cancer, Tumor Biol., 35, 10295-10300, https://doi.org/10.1007/s13277-014-2348-4.
Kainov, Y., Favorskaya, I., Delektorskaya, V., Chemeris, G., Komelkov, A., et al. (2014) CRABP1 provides high malignancy of transformed mesenchymal cells and contributes to the pathogenesis of mesenchymal and neuroendocrine tumors, Cell Cycle, 13, 1530-1539, https://doi.org/10.4161/cc.28475.
Rossetti, S., and Sacchi, N. (2019) 3D mammary epithelial cell models: a goldmine of dcis biomarkers and morphogenetic mechanisms, Cancers, 11, 130, https://doi.org/10.3390/cancers11020130.
Garattini, E., Bolis, M., Garattini, S. K., Fratelli, M., Centritto, F., et al. (2014) Retinoids and breast cancer: from basic studies to the clinic and back again, Cancer Treat. Rev., 40, 739-749, https://doi.org/10.1016/j.ctrv.2014.01.001.
Coyle, K. M., Dean, C. A., Thomas, M. L., Vidovic, D., Giacomantonio, C. A., et al. (2018) DNA methylation predicts the response of triple-negative breast cancers to all-trans retinoic acid, Cancers, 10, 397, https://doi.org/10.3390/cancers10110397.
Centritto, F., Paroni, G., Bolis, M., Garattini, S. K., Kurosaki, M., et al. (2015) Cellular and molecular determinants of all-trans retinoic acid sensitivity in breast cancer: luminal phenotype and RARα expression, EMBO Mol. Med., 7, 950-972, https://doi.org/10.15252/emmm.201404670.
Bolis, M., Garattini, E., Paroni, G., Zanetti, A., Kurosaki, M., et al. (2017) Network-guided modeling allows tumor-type independent prediction of sensitivity to all-trans-retinoic acid, Ann. Oncology, 28, 611-621, https://doi.org/10.1093/annonc/mdw660.
Coyle, K. M., Sultan, M., Thomas, M. L., Vaghar-Kashani, A., and Marcato, P. (2013) Retinoid signaling in cancer and its promise for therapy, J. Carcinogen. Mutagen., https://doi.org/10.4172/2157-2518.s7-006.
Miyake, T., Ueda, Y., Matsuzaki, S., Miyatake, T., Yoshino, K., et al. (2011) CRABP1-reduced expression is associated with poorer prognosis in serous and clear cell ovarian adenocarcinoma, J. Cancer Res. Clin. Oncol., 137, 715-722, https://doi.org/10.1007/s00432-010-0930-8.
Tanaka, K., Imoto, I., Inoue, J., Kozaki, K., Tsuda, H., et al. (2007) Frequent methylation-associated silencing of a candidate tumor-suppressor, CRABP1, in esophageal squamous-cell carcinoma, Oncogene, 26, 6456-6468, https://doi.org/10.1038/sj.onc.1210459.
Lind, G. E., Kleivi, K., Meling, G. I., Teixeira, M. R., Thiis-Evensen, E., et al. (2006) ADAMTS1, CRABP1, and NR3C1 identified as epigenetically deregulated genes in colorectal tumorigenesis, Cell. Oncol., 28, 259-272, https://doi.org/10.1155/2006/949506.
Wu, Q., Lothe, R. A., Ahlquist, T., Silins, I., Tropé, C. G., et al. (2007) DNA methylation profiling of ovarian carcinomas and their in vitro models identifies HOXA9, HOXB5, SCGB3A1, and CRABP1 as novel targets, Mol. Cancer, 6, 42, https://doi.org/10.1186/1476-4598-6-45.
Wang, F., Yang, Y., Fu, Z., Xu, N., Chen, F., et al. (2014) Differential DNA methylation status between breast carcinomatous and normal tissues, Biomed. Pharmacother., 68, 699-707, https://doi.org/10.1016/j.biopha.2014.07.014.
Stroganova, A. M., Chemeris, G. Yu., Chevkina, E. M., Senderovich, A., Karseladze, A. I. (2016) CRABP protein 1 and its role in the process of differentiation neuroblastoma, Vestnik RONTs im. N. N. Blokhina, 27, 157-163.
Bertucci, F., Houlgatte, R., Benziane, A., Granjeaud, S., Adélaïde, J., et al. (2000) Gene expression profiling of primary breast carcinomas using arrays of candidate genes, Hum. Mol. Genet., 9, 2981-2991, https://doi.org/10.1093/hmg/9.20.2981.
Tsibris, J. C. M., Segars, J., Coppola, D., Mane, S., Wilbanks, G. D., et al. (2002) Insights from gene arrays on the development and growth regulation of uterine leiomyomata, Fertil. Steril., 78, 114-121, https://doi.org/10.1016/S0015-0282(02)03191-6.
Fontana, J. A. (1992) Responses to retinoic acid of tamoxifen-sensitive and -resistant sublines of human breast cancer cell line MCF-7, Cancer Res., 52, 6164-6167.
Fontana, J. A. (1987) Interaction of retinoids and tamoxifen on the inhibition of human mammary carcinoma cell proliferation, Pathobiology, 55, 136-144, https://doi.org/10.1159/000163409.
Van der Leede, B. J. M., Folkers, G. E., van den Brink, C. E., van der Saag, P. T., and van der Burg, B. (1995) Retinoic acid receptor α1 isoform is induced by estradiol and confers retinoic acid sensitivity in human breast cancer cells, Mol. Cell. Endocrinol., 109, 77-86, https://doi.org/10.1016/0303-7207(95)03487-R.
Chlapek, P., Slavikova, V., Mazanek, P., Sterba, J., and Veselska, R. (2018) Why differentiation therapy sometimes fails: molecular mechanisms of resistance to retinoids, Int. J. Mol. Sci., 19, 132, https://doi.org/10.3390/ijms19010132.
Tari, A. M., Lim, S. J., Hung, M. C., Esteva, F. J., and Lopez-Berestein, G. (2002) Her2/neu induces all-trans retinoic acid (ATRA) resistance in breast cancer cells, Oncogene, 21, 5224-5232, https://doi.org/10.1038/sj.onc.1205660.
Wang, J., Guo, Y., Chu, H., Guan, Y., Bi, J., and Wang, B. (2013) Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis, Int. J. Mol. Sci., 14, 10015-10041, https://doi.org/10.3390/ijms140510015.
Gupta, A., Williams, B. R. G., Hanash, S. M., and Rawwas, J. (2006) Cellular retinoic acid-binding protein II is a direct transcriptional target of MycN in neuroblastoma, Cancer Res., 66, 8100-8108, https://doi.org/10.1158/0008-5472.CAN-05-4519.
Babuke, T., Ruonala, M., Meister, M., Amaddii, M., Genzler, C., et al. (2009) Hetero-oligomerization of reggie-1/flotillin-2 and reggie-2/flotillin-1 is required for their endocytosis, Cell. Signal., 21, 1287-1297, https://doi.org/10.1016/j.cellsig.2009.03.012.
Frick, M., Bright, N. A., Riento, K., Bray, A., Merrified, C., and Nichols, B. J. (2007) Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding, Curr. Biol., 17, 1151-1156, https://doi.org/10.1016/j.cub.2007.05.078.
Solis, G. P., Hoegg, M., Munderloh, C., Schrock, Y., Malaga-Trillo, E., et al. (2007) Reggie/flotillin proteins are organized into stable tetramers in membrane microdomains, Biochem. J., 403, 313-322, https://doi.org/10.1042/BJ20061686.
Funding
This study was financially supported by the Russian Foundation for Basic Research (project no. 19-015-00027A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Enikeev, A.D., Komelkov, A.V., Axelrod, M.E. et al. CRABP1 and CRABP2 Protein Levels Correlate with Each Other but Do Not Correlate with Sensitivity of Breast Cancer Cells to Retinoic Acid. Biochemistry Moscow 86, 217–229 (2021). https://doi.org/10.1134/S0006297921020103
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297921020103