Abstract
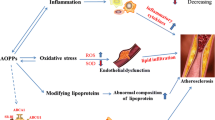
This review discusses formation of reactive halogen species (RHS) catalyzed by myeloperoxidase (MPO), an enzyme mostly present in leukocytes. An imbalance between the RHS production and body’s ability to remove or neutralize them leads to the development of halogenative stress. RHS reactions with proteins, lipids, carbohydrates, and antioxi-dants in the content of low-density lipoproteins (LDLs) of the human blood are described. MPO binds site-specifically to the LDL surface and modifies LDL properties and structural organization, which leads to the LDL conversion into proatherogenic forms captured by monocytes/macrophages, which causes accumulation of cholesterol and its esters in these cells and their transformation into foam cells, the basis of atherosclerotic plaques. The review describes the biomarkers of MPO enzymatic activity and halogenative stress, as well as the involvement of the latter in the development of atherosclerosis.
Similar content being viewed by others
Abbreviations
- ApoB100:
-
apolipoprotein B-100
- CP:
-
ceruloplas-min
- CVD:
-
cardiovascular disease
- HDL:
-
high-density lipopro-tein
- LDL:
-
low-density lipoprotein
- LDL-Br:
-
low-density lipoprotein modified by HOBr
- LDL-Cl:
-
low-density lipopro-tein modified by HOCl
- LPO:
-
lipid peroxidation
- mLDL:
-
modified low-density lipoprotein
- MPO:
-
myeloperoxidase
- RHS:
-
reactive halogen species
- VLDL:
-
very low-density lipoprotein
References
Klimov, A. N., and Nikulicheva, N. G. (1999) Lipids and Lipoproteins Metabolism and Its Defects [in Russian], Piter, St. Petersburg.
Anichkov, N. N. (1965) Basic Theoretical Principles for Further Studies of the Problem of Atherosclerosis. Atherosclerosis [in Russian], Meditsina, Leningrad, pp. 14–21.
Gofman, J. W., Glazier, F., Tamplin, A., Strisower, B., and De Lalla, O. (1954) Lipoproteins, coronary heart disease, and atherosclerosis, Physiol. Rev., 34, 589–607.
De Lalla, O. F., and Gofman, J. W. (1954) Ultracentrifugal analysis of serum lipoproteins, Methods Biochem. Anal., 1, 459–478.
Tada, H., Kawashiri, M. A., Nohara, A., Inazu, A., Mabuchi, H., and Yamagishi, M. (2017) Impact of clinical signs and genetic diagnosis of familial hypercholes-terolemia on the prevalence of coronary artery disease in patients with severe hypercholesterolemia, Eur. Heart J., 38, 1573–1579.
Carew, T. E. (1989) Role of biologically modified low-density lipoprotein in atherosclerosis, Am. J. Cardiol., 64, 18G–22G.
Aviram, M. (1993) Modified forms of low-density lipopro-tein and atherosclerosis, Atherosclerosis, 98, 1–9.
Brown, M. S., and Goldstein, J. L. (1976) Receptor-mediated control of cholesterol metabolism, Science, 191, 150–154.
Goldstein, J. L., Ho, Y. K., Basu, S. K., and Brown, M. S. (1979) Binding site on macrophages that mediates uptake and degradation of acetylated low-density lipoprotein, producing massive cholesterol deposition, Proc. Natl. Acad. Sci. USA, 76, 333–337.
Fogelman, A. M., Schechter, J. S., Hokom, M. Child, J. S., and Edwards, P. A. (1980) Malondialdehyde alteration of low-density lipoprotein leads to cholesterol accumulation in human monocyte-macrophages, Proc. Natl. Acad. Sci. USA, 77, 2214–2218.
Panasenko, O. M., Azizova, O. A., and Vladimirov, Yu. A. (1992) Peroxidation of blood lipoproteins and development of atherosclerosis, Sov. Med. Rev. B. Physicochem. Asp. Med., 3, 1–74.
Steinbrecher, U. P., Zhang, H., and Lougheed, M. (1990) Role of oxidatively modified LDL in atherosclerosis, Free Radic. Biol. Med., 9, 155–168.
Panasenko, O. M., Aksenov, D. V., Mel’nichenko, A. A., Suprun, I. V., Yanushevskaya, E. V., Vlasik, T. N., Sobenin, I. A., and Orekhov, A. N. (2005) Proteolysis of apoprotein B-100 impairs its topography on LDL surface and reduces LDL association resistance, Bull. Exp. Biol. Med., 140, 521–525.
Piha, M., Lindstedt, L., and Kovanen, P. T. (1995) Fusion of proteolyzed low-density lipoprotein in the fluid phase: a novel mechanism generating atherogenic lipoprotein particles, Biochemistry, 34, 10120–10129.
Aksenov, D. V., Mel’nichenko, A. A., Suprun, I. V., Yanushevskaya, E. V., Vlasik, T. N., Sobenin, I. A., Panasenko, O. M., and Orekhov, A. N. (2005) Phospholipid hydrolysis with phospholipases A2 and C impairs apolipoprotein B-100 conformation on the surface of low density lipoproteins by reducing their association resistance, Bull. Exp. Biol. Med., 140, 419–422.
Mel’nichenko, A. A., Tertov, V. V., Ivanova, O. A., Aksenov, D. V., Sobenin, I. A., Popov, E. V., Kaplun, V. V., Suprun, I. V., Panasenko, O. M., and Orekhov, A. N. (2005) Desialylation decreases the resistance of apoB-containing lipoproteins to aggregation and increases their atherogenic potential, Bull. Exp. Biol. Med., 140, 51–54.
Panasenko, O. M., Tertov, V. V., Melnichenko, A. A., Aksenov, D. V., Sobenin, I. A., Kaplun, V. V., Suprun, I. V., and Orekhov, A. N. (2006) Correlation between the athero-genic potential and particle size of apoB-containing lipoproteins deglycosylated with different enzymes, Biol. Membr. (Moscow), 23, 225–234.
Turk, Z., and Skrabalo, Z. (1987) The cell-interactive properties of glucosylated very-low-density and low-density lipoproteins, Cell Mol. Biol., 33, 345–354.
Avogaro, P., Bon, G. B., and Cazzolato, G. (1988) Presence of a modified low-density lipoprotein in humans, Arteriosclerosis, 8, 79–87.
Tertov, V. V., Sobenin, I. A., Gabbasov, Z. A., Popov, E. G., Jaakkola, O., Solakivi, T., Nikkari, T., Smirnov, V. N., and Orekhov, A. N. (1992) Multiple-modified desialylated low density lipoproteins that cause intracellular lipid accumulation. Isolation, fractionation and characterization, Labor. Invest., 67, 665–675.
Austin, M. A., Breslow, J. L., Hennekens, C. H., Buring, J. E., Willett, W. C., and Krauss, R. M. (1988) Low-density lipoprotein subclass patterns and risk of myocardial infarction, JAMA, 260, 1917–1921.
Daugherty, A., Dunn, J. L., Rateri, D. L., and Heinecke, J. W. (1994) Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions, J. Clin. Invest., 94, 437–444.
Schindhelm, R. K., van der Zwan, L. P., Teerlink, T., and Scheffer, P. G. (2009) Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin. Chem., 55, 1462–1470.
Klebanoff, S. J. (2005) Myeloperoxidase: friend and foe, J. Leukoc. Biol., 77, 598–625.
Panasenko, O. M., Gorudko, I. V., and Sokolov, A. V. (2013) Hypochlorous acid as a precursor of free radicals in living systems, Biochemistry (Moscow), 78, 1466–1489.
Panasenko, O. M., and Sergienko, V. I. (2010) Halogenative stress and its biomarkers, Vestn. Ross. Akad. Med. Nauk, No. 1, 22–39.
Sies, H. (1991) Role of reactive oxygen species in biological processes, Klin. Wochenschr., 69, 965–968.
Darley-Usmar, V., and Halliwell, B. (1996) Blood radicals: reactive nitrogen species, reactive oxygen species, transition metal ions, and the vascular system, Pharm. Res., 13, 649–662.
Fiedler, T. J., Davey, C. A., and Fenna, R. E. (2000) X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 Å resolution, J. Biol. Chem., 275, 11964–11971.
Schultz, J., and Kaminker, K. (1962) Myeloperoxidase of the leucocyte of normal human blood. 1. Content and localization, Arch. Biochem. Biophys., 96, 465–467.
Edwards, S. W. (1994) Biochemistry and Physiology of the Neutrophil, Cambridge University Press, Cambridge-New York-Melbourn, p. 299.
Deby-Dupont, G., Deby, C., and Lamy, M. (1999). Neutrophil myeloperoxidase revisited: its role in health and disease, Intensivmed, 36, 500–513.
Smith, R. J., Speziale, S. C., and Bowman, B. J. (1985) Properties of interleukin-1 as a complete secretogen for human neutrophils, Biochem. Biophys. Res. Commun., 130, 1233–1240.
Brinkmann, V., Reichard, U., Goosmann, C., Fauler, B., Uhlemann, Y., Weiss, D. S., Weinrauch, Y., and Zychlinsky, A. (2004) Neutrophil extracellular traps kill bacteria, Science, 303, 1532–1535.
Lau, D., Mollnau, H., Eiserich, J. P., Freeman, B. A., Daiber, A., Gehling, U. M., Brummer, J., Rudolph, V., Munzel, T., Heitzer, T., Meinertz, T., and Baldus, S. (2005) Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins, Proc. Natl. Acad. Sci. USA, 102, 431–436.
Grigorieva, D. V., Gorudko, I. V., Sokolov, A. V., Kostevich, V. A., Vasilyev, V. B., Cherenkevich, S. N., and Panasenko, O. M. (2016) Myeloperoxidase stimulates neu-trophil degranulation, Bull. Exp. Biol. Med., 161, 495–500.
Gorudko, I. V., Grigorieva, D. V., Sokolov, A. V., Shamova, E. V., Kostevich, V. A., Kudryavtsev, I. V., Syromiatnikova, E. D., Vasilyev, V. B., Cherenkevich, S. N., and Panasenko, O. M. (2018) Neutrophil activation in response to monomeric myeloperoxidase, Biochem. Cell Biol., 96, 592–601.
El Kebir, D., Jozsef, L., Pan, W., and Filep, J. G. (2008) Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation, Circ. Res., 103, 352–359.
Morris, J. C. (1966) The acid ionization constant of HOCl from 5 to 35°, J. Phys. Chem., 70, 3798–3805.
Furtmuller, P. G., Burner, U., and Obinger, C. (1998) Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate, Biochemistry, 37, 17923–17930.
Pattison, D. I., and Davies, M. J. (2006) Reactions of myeloperoxidase-derived oxidants with biological substrates: gaining chemical insight into human inflammatory diseases, Curr. Med. Chem., 13, 3271–3290.
Panasenko, O. M., Briviba, K., Klotz, L.-O., and Sies, H. (1997) Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrite, Arch. Biochem. Biophys, 343, 254–259.
Eiserich, J. P., Cross, C. E., Jones, A. D., Halliwell, B., and van der Vliet, A. (1996) Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification, J. Biol. Chem., 271, 19199–19208.
Byun, J., Mueller, M., Fabjan, J. S., and Heinecke, J. W. (1999) Nitrogen dioxide radical generated by the myeloper-oxidase-hydrogen peroxide-nitrite system promotes lipid peroxidation of low density lipoproteins, FEBS Lett., 455, 243–246.
Furtmuller, P. G., Jantschko, W., Zederbauer, M., Jakopitsch, C., Arnhold, J., and Obinger, C. (2004) Kinetics of interconversion of redox intermediates of lac-toperoxidase, eosinophil peroxidase and myeloperoxidase, Jpn. J. Infect. Dis., 57, S30–S31.
Kulinsky, V. I. (1999) Reactive oxygen forms and oxidative modification of macromolecules: benefit, harm and defense, Soros Educ. J., 1, 2–7.
Halliwell, B., and Gutteridge, J. M. C. (1999) Free Radical in Biology and Medicine, Oxford University Press, Oxford, pp. 617–783.
Gaut, J. P., Yeh, G. C., Tran, H. D., Byun, J., Henderson, J. P., Richter, G. M., Brennan, M. L., Lusis, A. J., Belaaouaj, A., Hotchkiss, R. S., and Heinecke, J. W. (2001) Neutrophils employ the myeloperoxidase system to generate antimicrobial brominating and chlorinating oxi-dants during sepsis, Proc. Natl. Acad. Sci. USA, 98, 11961–11966.
Dunford, H. B., and Marquez-Curtis, L. A. (2002) Myeloperoxidase: kinetic evidence for formation of enzyme-bound chlorinating intermediate, Methods Enzymol., 354, 338–350.
Thukkani, A. K., Albert, C. J., Wildsmith, K. R., Messner, M. C., Martinson, B. D., Hsu, F. F., and Ford, D. A. (2003) Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein, J. Biol. Chem., 278, 36365–36372.
Albert, C. J., Crowley, J. R., Hsu, F. F., Thukkani, A. K., and Ford, D. A. (2002) Reactive brominating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: disparate utilization of sodium halides in the production of alpha-halo fatty aldehydes, J. Biol. Chem., 277, 4694–4703.
Panasenko, O. M., Gorudko, I. V., Kovaleva, A. M., Gusev, S. A., Sergienko, V. I., and Matishev, D. G. (2010) Production and properties of reactive halogen species in carcinogenesis, Vestnik Uzhn. Nauch. Tsentra RAN, 6, 73–90.
Rudiger, J., Bobrowski, N., Liotta, M., and Hoffmann, T. (2017) Development and application of a sampling method for the determination of reactive halogen species in volcanic gas emissions, Anal. Bioanal. Chem., 409, 5975–5985.
Ronsein, G. E., Winterbourn, C. C., Di Mascio, P., and Kettle, A. J. (2014) Cross-linking methionine and amine residues with reactive halogen species, Free Radic. Biol. Med., 70, 278–287.
Pattison, D. I., and Davies, M. J. (2001) Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds, Chem. Res. Toxicol., 14, 1453–1464.
Pattison, D. I., and Davies, M. J. (2004) Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and use of 3-bro-motyrosine as a marker of oxidative stress, Biochemistry, 43, 4799–4809.
Malle, E., Marsche, G., Arnhold, J., and Davies, M. J. (2006) Modification of low-density lipoprotein by myeloper-oxidase-derived oxidants and reagent hypochlorous acid, Biochim. Biophys. Acta, 1761, 392–415.
Pattison, D. I., Hawkins, C. L., and Davies, M. J. (2003) Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis, and computational modeling, Chem. Res. Toxicol., 16, 439–449.
Skaff, O., Pattison, D. I., and Davies, M. J. (2007) Kinetics of hypobromous acid-mediated oxidation of lipid components and antioxidants, Chem. Res. Toxicol., 20, 1980–1988.
Hazell, L. J., van den Berg, J. J., and Stocker, R. (1994) Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation, Biochem. J., 302, 297–304.
Jerlich, A., Horakova, L., Fabjan, J. S., Giessauf, A., Jurgens, G., and Schaur, R. J. (1999) Correlation of low-density lipoprotein modification by myeloperoxidase with hypochlorous acid formation, Int. J. Clin. Lab. Res., 29, 155–161.
Yang, C. Y., Gu, Z. W., Yang, M., Lin, S. N., Garcia-Prats, A. J., Rogers, L. K., Welty, S. E., and Smith, C. V. (1999) Selective modification of ApoB100 in the oxidation of low density lipoproteins by myeloperoxidase in vitro, J. Lipid Res., 40, 686–698.
Delporte, C., Boudjeltia, K. Z., Noyon, C., Furtmuller, P. G., Nuyens, V., Slomianny, M. C., Madhoun, P., Desmet, J. M., Raynal, P., Dufour, D., Koyani, C. N., Reye, F., Rousseau, A., Vanhaeverbeek, M., Ducobu, J., Michalski, J. C., Neve, J., Vanhamme, L., Obinger, C., Malle, E., and Van Antwerpen, P. (2014) Impact of myeloperoxidase-LDL interactions on enzyme activity and subsequent post-translational oxidative modifications of ApoB100, J. Lipid Res., 55, 747–757.
Hawkins, C. L., and Davies, M. J. (2005) The role of reactive N-bromo species and radical intermediates in hypo-bromous acid-induced protein oxidation, Free Radic. Biol. Med., 9, 900–912.
Hawkins, C. L., Pattison, D. I., and Davies, M. J. (2003) Hypochlorite-induced oxidation of amino acids, peptides and proteins, Amino Acids, 25, 259–274.
Fu, X., Mueller, D. M., and Heinecke, J. W. (2002) Generation of intramolecular and intermolecular sulfe-namides, sulfinamides, and sulfonamides by hypochlorous acid: a potential pathway for oxidative cross-linking of low-density lipoprotein by myeloperoxidase, Biochemistry, 41, 1293–1301.
Heinecke, J. W., Li, W., Francis, G. A., and Goldstein, J. A. (1993) Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins, J. Clin. Invest., 91, 2866–2872.
Thomas, E. L. (1979) Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli, Infect. Immun., 23, 522–531.
Hawkins, C. L., and Davies, M. J. (1998) Reaction of HOCl with amino acids and peptides: EPR evidence for rapid rearrangement and fragmentation reactions of nitrogen-centered radicals. J. Chem. Soc. Perkin Trans., 2, 1937–1945.
Podrez, E. A., Febbraio, M., Sheibani, N., Schmitt, D., Silverstein, R. L., Hajjar, D. P., Cohen, P. A., Frazier, W. A., Hoff, H. F., and Hazen, S. L. (2000) Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species, J. Clin. Invest., 105, 1095–1108.
Panasenko, O. M., Arnhold, J., and Sergienko, V. I. (2002) Impairment of membrane lipids by hypochlorite, Biol. Membr. (Moscow), 19, 403–434.
Panasenko, O. M., Arnhold, J., Sergienko, V. I., Arnold, K., and Vladimirov, Yu. A. (1996) Stoichiometry of hypochlorite interaction with unsaturated bonds of phos-phatidylcholine and free fatty acids in liposomes, Membr. Cell Biol., 10, 291–302.
Winterbourn, C. C., van den Berg, J. J., Roitman, E., and Kuypers, F. A. (1992) Chlorohydrin formation from unsat-urated fatty acids reacted with hypochlorous acid, Arch. Biochem. Biophys., 296, 547–555.
Spalteholz, H., Panasenko, O. M., and Arnhold, J. (2006) Formation of reactive halide species by myeloperoxidase and eosinophil peroxidase, Arch. Biochem. Biophys., 445, 225–234.
Panasenko, O. M., Vakhrusheva, T., Tretyakov, V., Spalteholz, H., and Arnhold, J. (2007) Influence of chloride on modification of unsaturated phosphatidylcholines by the myeloperoxidase/hydrogen peroxide/bromide system, Chem. Phys. Lipids, 149, 40–51.
Carr, A. C., Winterbourn, C. C., and van den Berg, J. J. (1996) Peroxidase-mediated bromination of unsaturated fatty acids to form bromohydrins, Arch. Biochem. Biophys., 327, 227–233.
Panasenko, O. M., Spalteholz, H., Schiller, J., and Arnhold, J. (2006) Leukocytic myeloperoxidase-mediated formation of bromohydrins and lysophospholipids from unsaturated phosphatidylcholines, Biochemistry (Moscow), 71, 571–580.
Panasenko, O. M., Osipov, A. N., Schiller, J., and Arnhold, J. (2002) Interaction of exogenous hypochlorite or hypochlorite produced by myeloperoxidase + H2O2 + Cl- system with unsaturated phosphatidylcholines, Biochemistry (Moscow), 67, 889–900.
Panasenko, O. M., Spalteholz, H., Schiller, J., and Arnhold, J. (2003). Myeloperoxidase-induced formation of chlorohydrins and lysophospholipids from unsaturated phosphatidylcholines, Free Radic. Biol. Med., 34, 553–562.
Panasenko, O. M., Spalteholz, H., Schiller, J., and Arnhold, J. (2004) Myeloperoxidase mediated destruction of unsaturated phosphatidylcholines in liposomes, Biol. Membr. (Moscow), 21, 138–150.
Spalteholz, H., Wenske, K., Panasenko, O. M., Schiller, J., and Arnhold, J. (2004) Evaluation of products upon the reaction of hypochlorous acid with unsaturated phos-phatidylcholines, Chem. Phys. Lipids, 129, 85–96.
Arnhold, J., Osipov, A. N., Spalteholz, H., Panasenko, O. M., and Schiller, J. (2002) Formation of lysophospholipids from unsaturated phosphatidylcholines under the influence of hypochlorous acid, Biochim. Biophys. Acta, 1572, 91–100.
Arnhold, J., Osipov, A. N., Spalteholz, H., Panasenko, O. M., and Schiller, J. (2001) Effects of hypochlorous acid on unsaturated phosphatidylcholines, Free Radic. Biol. Med., 31, 1111–1119.
Engelmann, B., Brautigam, C., and Thiery, J. (1994) Plasmalogen phospholipids as potential protectors against lipid peroxidation of low density lipoproteins, Biochem. Biophys. Res. Commun., 204, 1235–1242.
Albert, C. J., Crowley, J. R., Hsu, F. F., Thukkani, A. K., and Ford, D. A. (2001) Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens. Identification of 2-chlorohexadecanal, J. Biol. Chem., 276, 23733–23741.
Leßig, J., Schiller, J., Arnhold, J., and Fuchs, B. (2007) Hypochlorous acid-mediated generation of glycerophos-phocholine from unsaturated plasmalogen glycerophos-phocholine lipids, J. Lipid Res., 48, 1316–1324.
Messner, M. C., Albert, C. J., Hsu, F., and Ford, D. A. (2006) Selective plasmenylcholine oxidation by hypo-chlorous acid: formation of lysophosphatidylcholine chlorohydrins, Chem. Phys. Lipids, 144, 34–44.
Skaff, O., Pattison, D. I., and Davies, M. J. (2008) The vinyl ether linkages of plasmalogens are favored targets for myeloperoxidase-derived oxidants: a kinetic study, Biochemistry, 47, 8237–8245.
Schiller, J., Zschornig, O., Petkovic, M., Muller, M., Arnhold, J., and Arnold, K. (2001) Lipid analysis of human HDL and LDL by MALDI-TOF mass spectrometry and 31P-NMR, J. Lipid Res., 42, 1501–1508.
Zakiev, E. R., Sukhorukov, V. N., Melnichenko, A. A., Sobenin, I. A., Ivanova, E. A., and Orekhov, A. N. (2016) Lipid composition of circulating multiple-modified low-density lipoprotein, Lipids Health Dis., 15, 134.
Thukkani, A. K., McHowat, J., Hsu, F. F., Brennan, M. L., Hazen, S. L., and Ford, D. A. (2003) Identification of alpha-chloro fatty aldehydes and unsaturated lysophos-phatidylcholine molecular species in human atherosclerotic lesions, Circulation, 108, 3128–3133.
Siess, W., Zangl, K. J., Essler, M., Bauer, M., Brandl, R., Corrinth, C., Bittman, R., Tigyi, G., and Aepfelbacher, M. (1999) Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions, Proc. Nat. Acad. Sci. USA, 96, 6931–6936.
Nusshold, C., Kollroser, M., Kofeler, H., Rechberger, G., Reicher, H., Ullen, A., Bernhart, E., Waltl, S., Kratzer, I., Hermetter, A., Hackl, H., Trajanoski, Z., Hrzenjak, A., Malle, E., and Sattler, W. (2010) Hypochlorite modification of sphingomyelin generates chlorinated lipid species that induce apoptosis and proteome alterations in dopaminergic PC12 neurons in vitro, Free Radic. Biol. Med., 48, 1588–600.
Spalteholz, H., Wenske, K., and Arnhold, J. (2005) Interaction of hypohalous acids and heme peroxidases with unsaturated phosphatidylcholines, Biofactors, 24, 67–76.
Heinecke, J. W., Li, W., Mueller, D. M., Bohrer, A., and Turk, J. (1994) Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: potential markers for lipoproteins oxidatively damaged by phagocytes, Biochemistry, 33, 10127–10136.
Carr, A. C., van den Berg, J. J., and Winterbourn, C. C. (1996) Chlorination of cholesterol in cell membranes by hypochlorous acid, Arch. Biochem. Biophys., 332, 63–69.
Hazen, S. L., Hsu, F. F., Duffin, K., and Heinecke, J. W. (1996) Molecular chlorine generated by the myeloperoxi-dase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols, J. Biol. Chem., 271, 23080–23088.
Van den Berg, J. J., Winterbourn, C. C., and Kuypers, F. A. (1993) Hypochlorous acid-mediated modification of cholesterol and phospholipid: analysis of reaction products by gas chromatography-mass spectrometry, J. Lipid Res., 34, 2005–2012.
Sergienko, V. I., Panasenko, O. M., and Murina, M. A. (1999) Development and introduction of electrochemical methods of detoxication in medicine, Efferent. Ter., 5,No.4, 8–17.
Vissers, M. C., Carr, A. C., and Chapman, A. L. (1998) Comparison of human red cell lysis by hypochlorous and hypobromous acids: insights into the mechanism of lysis, Biochem. J., 330, 131–138.
Panasenko, O. M. (1997). The mechanism of the hypochlorite-induced lipid peroxidation, Biofactors, 6, 181–190.
Stelmaszynska, T., Kukovetz, E., Egger, G., and Schaur, R. J. (1992) Possible involvement of myeloperoxidase in lipid peroxidation, Int. J. Biochem., 24, 121–128.
Evgina, S. A., Panasenko, O. M., Sergienko, V. I., and Vladimirov, Yu. A. (1992) Peroxidation of human plasma lipoproteins induced by hypochlorite, Biol. Membr. (Moscow), 6, 1247–1254.
Hazell, L. J., Davies, M. J., and Stocker, R. (1999) Secondary radicals derived from chloramines of apolipoprotein B-100 contribute to HOCl-induced lipid peroxidation of low-density lipoproteins, Biochem. J., 339, 489–495.
Panasenko, O. M., Evgina, S. A., Aidyraliev, R. K., Sergienko, V. I., and Vladimirov, Yu. A. (1994) Peroxidation of human blood lipoproteins induced by exogenous hypochlorite or hypochlorite generated in the system of “myeloperoxidase + H2O2 + Cl-”, Free Radic. Biol. Med., 16, 143–148.
Panasenko, O. M., Evgina, S. A., Driomina, E. S., Sharov, V. S., Sergienko, V. I., and Vladimirov, Yu. A. (1995) Hypochlorite induces lipid peroxidation in blood lipopro-teins and phospholipid liposomes, Free Radic. Biol. Med., 19, 133–140.
Panasenko, O. M., Arnhold, J., Arnhold, K., Vladimirov, Iu. A., and Sergienko, V. I. (1996) Comparative study of the kinetics of phospholipid liposome peroxidation, induced by hypochlorite and in the Fe2+ + ascorbate system, Biofizika, 41, 334–341.
Gorbatenkova, E. A., Artmann, G. M., and Panasenko, O. M. (2000) Hypochlorous acid and human blood low density lipoproteins modified by hypochlorous acid increase erythrocyte adhesion to endothelial cells, Membr. Cell Biol., 13, 537–546.
Panasenko, O. M., Evgina, S. A., and Sergienko, V. I. (1993) Capacity of hypochlorite to penetrate into the lipid phase of human blood lipoproteins, Bull. Exp. Biol. Med., 115, 358–360.
Panasenko, O. M., Panasenko, O. O., Briviba, K., and Sies, H. (1997) Hypochlorite destroys carotenoids in low density lipoproteins thus decreasing their resistance to per-oxidative modification, Biochemistry (Moscow), 62, 1140–1145.
Chekanov, A. V., Osipov, A. N., Vladimirov, Iu. A., Sergienko, V. I., and Panasenko, O. M. (2007) A comparative spin trapping study of hypobromous and hypochlorous acids interaction with tert-butyl hydroperoxide, Biofizika, 52, 5–13.
Rice-Evans, C., Leake, D., Bruckdorfer, K. R., and Diplock, A. T. (1996) Practical approaches to low density lipoprotein oxidation: whys, wherefores and pitfalls, Free Radic. Res., 25, 285–311.
Osipov, A. N., Panasenko, O. M., Chekanov, A. V., and Arnhold, J. (2002) Interaction of tert-butyl hydroperoxide with hypochlorous acid. A spin trapping and chemilumi-nescence study, Free Radic. Res., 36, 749–754.
Panasenko, O. M., Osipov, A. N., Chekanov, A. V., Arnhold, J., and Sergienko, V. I. (2002) Peroxyl radical is produced upon the interaction of hypochlorite with tert-butyl hydroperoxide, Biochemistry (Moscow), 67, 880–888.
Chekanov, A. V., Panasenko, O. M., Osipov, A. N., Arnhold, J., Kazarinov, K. D., Vladimirov, Iu. A., and Sergienko, V. I. (2002) Interaction of tert-butyl hydroperoxide with hypochlorite induces peroxyl radicals. A chemiluminescence study, Biofizika, 47, 787–794.
Chekanov, A. V., Panasenko, O. M., Osipov, A. N., Matveeva, N. S., Kazarinov, K. D., Vladimirov, Iu. A., and Sergienko, V. I. (2005) The interaction of hypochlorite with fatty acid hydroperoxides results in the generation of free radicals, Biofizika, 50, 13–19.
Panasenko, O. M., Chekanov, A. V., Arnhold, J., Sergienko, V. I., Osipov, A. N., and Vladimirov, Y. A. (2005) Generation of free radicals during decomposition of hydroperoxide in the presence of myeloperoxidase or activated neutrophils, Biochemistry (Moscow), 70, 998–1004.
Chekanov, A. V., Osipov, A. N., Vladimirov, Yu. A., Sergienko, V. I., and Panasenko, O. M. (2006) Interaction of hypobromite with tert-butyl hydroperoxide. Role in phospholipid liposome peroxidation, Biol. Membr. (Moscow), 23, 426–432.
Noguchi, N., Nakada, A., Itoh, Y., Watanabe, A., and Niki, E. (2002) Formation of active oxygen species and lipid peroxidation induced by hypochlorite, Arch. Biochem. Biophys., 397, 440–447.
Miyamoto, S., Martinez, G. R., Rettori, D., Augusto, O., Medeiros, M. H. G., and Di Mascio, P. (2006) Linoleic acid hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen, Proc. Natl. Acad. Sci. USA, 103, 293–298.
Crisby, M., Henareh, L., and Agewall, S. (2014) Relationship between oxidized LDL, IgM, and IgG autoantibodies to ox-LDL levels with recurrent cardiovascular events in Swedish patients with previous myocardial infarction, Angiology, 65, 932–936.
Holvoet, P., Vanhaecke, J., Janssens, S., Van de Werf, F., and Collen, D. (1998) Oxidized LDL and malondialde-hyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease, Circulation, 98, 1487–1489.
Osipov, A. N., Briukhanova, E. V., Vakhrusheva, T. V., Panasenko, O. M., and Vladimirov, Iu. A. (1997) Effect of hypochlorite and hydrogen peroxide on the ability of hemoglobin to stimulate lipid peroxidation of low-density lipoproteins, Biofizika, 42, 400–407.
Yakutova, E. Sh., Dremina, Ye. S., Yevgina, S. A., Osipov, A. N., Sharov, V. S., Panasenko, O. M., and Vladimirov, Yu. A. (1994) Formation of free radicals on interaction of hypochlorite with iron (II) ions, Biophysics, 39, 241–245.
Savenkova, M. L., Mueller, D. M., and Heinecke, J. W. (1994) Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid peroxidation in low density lipoprotein, J. Biol. Chem., 269, 20394–20400.
Kawai, Y., Kiyokawa, H., Kimura, Y., Kato, Y., Tsuchiya, K., and Terao, J. (2006) Hypochlorous acid-derived modification of phospholipids: characterization of aminophos-pholipids as regulatory molecules for lipid peroxidation, Biochemistry, 45, 14201–14211.
Arnhold, J., Panasenko, O. M., Schiller, J., Vladimirov, Yu. A., and Arnold, K. (1995) The action of hypochlorous acid on phosphatidylcholine liposomes in dependence on the content of double bonds. Stoichiometry and NMR analysis, Chem. Phys. Lipids, 78, 55–64.
Panasenko, O. M., Arnhold, J., Vladimirov, Yu. A., Arnold, K., and Sergienko, V. I. (1997) Hypochlorite-induced peroxidation of egg yolk phosphatidylcholine is mediated by hydroperoxides, Free Radic. Res., 27, 1–12.
Richter, G., Schober, C., Suß, R., Fuchs, B., Birkemeyer, C., and Schiller, J. (2008) Comparison of the positive and negative ion electrospray ionization and matrix-assisted laser desorption ionization-time-of-flight mass spectra of the reaction products of phosphatidylethanolamines and hypochlorous acid, Anal. Biochem., 376, 157–159.
Carr, A. C., van den Berg, J. J., and Winterbourn, C. C. (1998) Differential reactivities of hypochlorous and hypo-bromous acids with purified Escherichia coli phospholipid: formation of haloamines and halohydrins, Biochim. Biophys. Acta, 1392, 254–264.
Tertov, V. V., Orekhov, A. N., Sobenin, I. A., Morrisett, J. D., Gotto, A. M., and Guevara, J. G., (1993) Carbohydrate composition of protein and lipid components in sialic acid-rich and -poor low-density lipoproteins from subjects with and without coronary artery disease, J. Lipid Res., 34, 365–375.
Taniguchi, T., Ishikawa, Y., Tsunemitsu, M., and Fukuzaki, H. (1989) The structures of asparagine-linked sugar chains of human apolipoprotein B-100, Arch. Biochem. Biophys., 273, 197–205.
Prutz, W. A. (1996) Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates, Arch. Biochem. Biophys., 332, 110–120.
Schiller, J., Arnhold, J., and Arnold, K. (1995) Action of hypochlorous acid on polymeric components of cartilage. Use of 13C NMR spectroscopy, Z. Naturforsch., 50, 721–728.
Schiller, J., Arnhold, J., and Arnold, K. (1995) NMR studies of the action of hypochlorous acid on native pig articular cartilage, Eur. J. Biochem., 233, 672–676.
Hawkins, C. L., and Davies, M. J. (1998) Degradation of hyaluronic acid, poly- and monosaccharides, and model compounds by hypochlorite: evidence for radical intermediates and fragmentation, Free Radic. Biol. Med., 24, 1396–1410.
Rees, M. D., Hawkins, C. L., and Davies, M. J. (2003) Hypochlorite-mediated fragmentation of hyaluronan, chondroitin sulfates, and related N-acetyl glycosamines: evidence for chloramide intermediates, free radical transfer reactions, and site-specific fragmentation, J. Am. Chem. Soc., 125, 13719–13733.
Rees, M. D., Mcniven, T. N., and Davies, M. J. (2007) Degradation of extracellular matrix and its components by hypobromous acid, Biochem. J., 401, 587–596.
Rees, M. D., Hawkins, C. L., and Davies, M. J. (2004) Hypochlorite and superoxide radicals can act synergisti-cally to induce fragmentation of hyaluronan and chon-droitin sulphates, Biochem. J., 381, 175–184.
Rees, M. D., and Davies, M. J. (2006) Heparan sulfate degradation via reductive homolysis of its N-chloro-deriv-atives, Am. Chem. Soc., 128, 3085–3097.
Tertov, V. V., Kaplun, V. V., Sobenin, I. A., and Orekhov, A. N. (1998) Low-density lipoprotein modification occurring in human plasma. Possible mechanism of in vivo lipopro-tein desialylation as a primary step of atherogenic modification, Atherosclerosis, 138, 183–195.
Sukhorukov, V. N., Karagodin, V. P., and Orekhov, A. N. (2016) Atherogenic modification of low-density lipopro-teins, Biomed. Khim., 62, 391–402.
Tertov, V. V., Nikonova, E. Y., Nifant’ev, N. E., Bovin, N. V., and Orekhov, A. N. (2002) Human plasma trans-siali-dase donor and acceptor specificity, Biochemistry (Moscow), 67, 908–913.
Estrbauer, H., Puhl, H., Waeg, G., Krebs, A., Tatzber, F., and Rabol, H. (1993) Vitamin E and atherosclerosis - an overview, in Vitamin E (Mino, M., Nakamura, H., Diplock, A. T., and Keyden, H. J., eds.) Japan Scientific Societies Press, Tokyo, pp. 233–241.
Hazell, L. J., and Stocker, R. (1993) Oxidation of low-density lipoprotein with hypochlorite causes transformation of the lipoprotein into a high-uptake form for macrophages, Biochem. J., 290, 165–172.
Albrich, J. M., McCarthy, C. A., and Hurst, J. K. (1981) Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase, Proc. Natl. Acad. Sci. USA, 78, 210–214.
Hazell, L. J., and Stocker, R. (1997) a-Tocopherol does not inhibit hypochlorite-induced oxidation of apolipopro-tein B-100 of low-density lipoprotein, FEBS Lett., 414, 541–544.
Panasenko, O. M., Evgina, S. A., and Sergienko, V. I. (1993) A spin-probe study of the structural change in human blood lipoproteins under the action of sodium hypochlorite, Bull. Exp. Biol. Med., 116, 153–155.
Panasenko, O. M. (1998) Hypochlorite and Oxidative Modification of Human Blood Lipoproteins: Ph. D. Thesis, Biological Sciences [in Russian], Research Institute of Physico-Chemical Medicine, Moscow.
Panasenko, O. M., Borin, M. L., Azizova, O. A., and Arnol’d, K. (1985) Determination of the surface charge of lipoproteins and its changes during lipid peroxidation, Biofizika, 30, 822–827.
Panasenko, O. M., Evgina, S. A., Nikitin, S. V., and Sergienko, V. I. (1995) The effect of hypochlorite on electrical surface properties of human blood lipoproteins, Biofizika, 40, 545–550.
Jerlich, A., Fabjan, J. S., Tschabuschnig, S., Smirnova, A. V., Horakova, L., Hayn, M., Auer, H., Guttenberger, H., Leis, H. J., Tatzber, F., Waeg, G., and Schaur, R. J. (1998) Human low density lipoprotein as a target of hypochlorite generated by myeloperoxidase, Free Radic. Biol. Med., 24, 1139–1148.
Panasenko, O. M., Melnichenko, A. A., Aksenov, D. V., Vakhrusheva, T. V., Suprun, I. V., Yanushevskaya, E. V., Vlasik, T. N., Sobenin, I. A., and Orekhov, A. N. (2004) Myeloperoxidase increases atherogenic potential of human blood low density lipoproteins by modifying their surface and lowering the resistance to association, Biol. Membr. (Moscow), 21, 498–505.
Guyton, J. R., and Klemp, K. F. (1994) Development of the atherosclerotic core region. Chemical and ultrastructural analysis of microdissected atherosclerotic lesions from human aorta, Arterioscler. Thromb., 14, 1305–1314.
Tirziu, D., Dobrian, A., Tasca, C., Simionescu, M., and Simionescu, N. (1995) Intimal thickenings of human aorta contain modified reassembled lipoproteins, Atherosclerosis, 112, 101–114.
O’Connell, A. M., Gieseg, S. P., and Stanley, K. K. (1994) Hypochlorite oxidation causes cross-linking of Lp(a), Biochim. Biophys. Acta, 1225, 180–186.
Panasenko, O. M., Azizova, O. A., Arnold, K., and Borin, M. L. (1987) Alterations of surface charges of plasma lipoproteins in ischemic heart disease, Biomed. Biochim. Acta, 46, 97–102.
Carr, A. C., Myzak, M. C., Stocker, R., McCall, M. R., and Frei, B. (2000) Myeloperoxidase binds to low-density lipoprotein: potential implications for atherosclerosis, FEBS Lett., 487, 176–180.
Sokolov, A. V., Chekanov, A. V., Kostevich, V. A., Aksenov, D. V., Vasilyev, V. B., and Panasenko, O. M. (2011) Revealing binding sites for myeloperoxidase on the surface of human low density lipoproteins, Chem. Phys. Lipids, 164, 49–53.
Sokolov, A. V., Ageeva, K. V., Cherkalina, O. S., Pulina, M. O., Zakharova, E. T., Prozorovskii, V. N., Aksenov, D. V., Vasilyev, V. B., and Panasenko, O. M. (2010) Identification and properties of complexes formed by myeloperoxidase with lipoproteins and ceruloplasmin, Chem. Phys. Lipids, 163, 347–355.
Sokolov, A. V., Kostevich, V. A., Runova, O. L., Gorudko, I. V., Vasilyev, V. B., Cherenkevich, S. N., and Panasenko, O. M. (2014) Proatherogenic modification of LDL by surface-bound myeloperoxidase, Chem. Phys. Lipid, 180, 72–80.
Segelmark, M., Persson, B., Hellmark, T., and Wieslander, J. (1997) Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin. Exp. Immunol., 108, 167–174.
Sokolov, A. V., Ageeva, K. V., Pulina, M. O., Cherkalina, O. S., Samygina, V. R., Vlasova, I. I., Panasenko, O. M., Zakharova, E. T., and Vasilyev, V. B. (2008) Ceruloplasmin and myeloperoxidase in complex affect the enzymatic properties of each other, Free Radic. Res., 42, 989–998.
Panasenko, O. M., Chekanov, A. V., Vlasova, I. I., Sokolov, A. V., Ageeva, K. V., Pulina, M. O., Cherkalina, O. S., and Vasil’ev, V. B. (2008) A study of the effect of ceruloplasmin and lactoferrin on the chlorination activity of leukocytic myeloperoxidase using the chemiluminescence method, Biophysics, 53, 268–272.
Karakas, M., and Koenig, W. (2012) Myeloperoxidase production by macrophage and risk of atherosclerosis, Curr. Atheroscler. Rep., 14, 277–283.
Zhang, R., Brennan, M. L., Fu, X., Aviles, R. J., Pearce, G. L., Penn, M. S., Topol, E. J., Sprecher, D. L., and Hazen, S. L. (2001) Association between myeloperoxidase levels and risk of coronary artery disease, JAMA, 286, 2136–2142.
Chen, L. Q., Rohatgi, A., Ayers, C. R., Das, S. R., Khera, A., Berry, J. D., McGuire, D. K., de and Lemos, J. A. (2011) Race-specific associations of myeloperoxidase with atherosclerosis in a population-based sample: the Dallas Heart Study, Atherosclerosis, 219, 833–838.
Karakas, M., Koenig, W., Zierer, A., Herder, C., Rottbauer, W., Baumert, J., Meisinger, C., and Thorand, B. (2012) Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg study, J. Intern. Med., 271, 43–50.
Sugiyama, S., Okada, Y., Sukhova, G. K., Virmani, R., Heinecke, J. W., and Libby, P. (2001) Macrophage myeloperoxidase regulation by granulocyte macrophage colony-stimulating factor in human atherosclerosis and implications in acute coronary syndromes, Am. J. Pathol., 158, 879–891.
Cheng, M. L., Chen, C. M., Gu, P. W., Ho, H. Y., and Chiu, D. T. (2008) Elevated levels of myeloperoxidase, white blood cell count and 3-chlorotyrosine in Taiwanese patients with acute myocardial infarction, Clin. Biochem., 41, 554–560.
Ho, H. Y., Cheng, M. L., Chen, C. M., Gu, P. W., Wang, Y. L., Li, J. M., and Chiu, D. T. (2008) Oxidative damage markers and antioxidants in patients with acute myocardial infarction and their clinical significance, Biofactors, 34, 135–145.
Hazen, S. L., and Heinecke, J. W. (1997) 3-Chlorotyrosine, a specific marker of myeloperoxidase-cat-alyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima, J. Clin. Invest., 99, 2075–2081.
Hazen, S. L., Crowley, J. R., Mueller, D. M., and Heinecke, J. W. (1997) Mass spectrometric quantification of 3-chlorotyrosine in human tissues with attomole sensitivity: a sensitive and specific marker for myeloperoxidase-catalyzed chlorination at sites of inflammation, Free Radic. Biol. Med., 23, 909–916.
Bergt, C., Pennathur, S., Fu, X., Byun, J., O’Brien, K., McDonald, T. O., Singh, P., Anantharamaiah, G. M., Chait, A., Brunzell, J., Geary, R. L., Oram, J. F., and Heinecke, J. W. (2004) The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport, Proc. Natl. Acad. Sci. USA, 101, 13032–13037.
Pennathur, S., Bergt, C., Shao, B., Byun, J., Kassim, S. Y., Singh, P., Green, P. S., McDonald, T. O., Brunzell, J., Chait, A., Oram, J. F., O’brien, K., Geary, R. L., and Heinecke, J. W. (2004) Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species, J. Biol. Chem., 279, 42977–42983.
Zheng, L., Nukuna, B., Brennan, M. L., Sun, M., Goormastic, M., Settle, M., Schmitt, D., Fu, X., Thomson, L., Fox, P. L., Ischiropoulos, H., Smith, J. D., Kinter, M., and Hazen, S. L. (2004) Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease, J. Clin. Invest., 114, 529–541.
Rudolph, V., Andrie, R. P., Rudolph, T. K., Friedrichs, K., Klinke, A., Hirsch-Hoffmann, B., Schwoerer, A. P., Lau, D., Fu, X., Klingel, K., Sydow, K., Didie, M., Seniuk, A., von Leitner, E. C., Szoecs, K., Schrickel, J. W., Treede, H., Wenzel, U., Lewalter, T., Nickenig, G., Zimmermann, W. H., Meinertz, T., Boger, R. H., Reichenspurner, H., Freeman, B. A., Eschenhagen, T., Ehmke, H., Hazen, S. L., Willems, S., and Baldus, S. (2010) Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation, Nat. Med., 16, 470–474.
Malle, E., Hazell, L., Stocker, R., Sattler, W., Esterbauer, H., and Waeg, G. (1995) Immunologic detection and measurement of hypochlorite-modified LDL with specific monoclonal antibodies, Arterioscler. Thromb. Vasc. Biol., 15, 982–989.
Hazell, L. J., Arnold, L., Flowers, D., Waeg, G., Malle, E., and Stocker, R. (1996) Presence of hypochlorite-mod-ified proteins in human atherosclerotic lesions, J. Clin. Invest., 97, 1535–1544.
Hazell, L. J., Baernthaler, G., and Stocker, R. (2001) Correlation between intima-to-media ratio, apolipopro-tein B-100, myeloperoxidase, and hypochlorite-oxidized proteins in human atherosclerosis, Free Radic. Biol. Med., 31, 1254–1262.
Jerlich, A., Pitt, A. R., Schaur, R. J., and Spickett, C. M. (2000) Pathways of phospholipid oxidation by HOCl in human LDL detected by LC-MS, Free Radic. Biol. Med., 28, 673–682.
Messner, M. C., Albert, C. J., McHowat, J., and Ford, D. A. (2008) Identification of lysophosphatidylcholine-chlorohydrin in human atherosclerotic lesions, Lipids, 43, 243–249.
Domigan, N. N., Carr, A. C., Lewis, J. G., Elder, P. A., and Winterbourn, C. C. (1997) A monoclonal antibody recognizing the chlorohydrin derivates of oleic acid for probing hypochlorous acid involvement in tissue injury, Redox Rep., 3, 111–117.
Thukkani, A. K., Martinson, B. D., Albert, C. J., Vogler, G. A., and Ford, D. A. (2005) Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury, Am. J. Physiol. Heart Circ. Physiol., 288, H2955–H2964.
Takeshita, J., Byun, J., Nhan, T. Q., Pritchard, D. K., Pennathur, S., Schwartz, S. M., Chait, A., and Heinecke, J. W. (2006) Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages, J. Biol. Chem., 281, 3096–3104.
Sokolov, A. V., Kostevich, V. A., Vasilyev, V. B., and Panasenko, O. M. (2018) Hypohalous acids modified lipoproteins as biomarkers of halogenative stress at cardiovascular diseases, in Molecular, Membrane and Cellular Basis of Biosystem Functioning (Volotovsky, I. D., ed.) Izdvo BGU, Minsk, p. 171.
Sokolov, A. V., Kostevich, V. A., Gorbunov, N. V., Grigorieva, D. V., Gorudko, I. V., Vasilyev, V. B., and Panasenko, O. M. (2018) A link between active myeloper-oxidase and chlorinated ceruloplasmin in blood plasma of patients with cardiovascular diseases, Med. Immunol. (Russia), 20, 699–710.
Liu, L., Wang, Y. X., Zhou, J., Long, F., Sun, H. W., Liu, Y., Chen, Y. Z., and Jiang, C. L. (2005) Rapid non-genomic inhibitory effects of glucocorticoids on human neutrophil degranulation, Inflamm. Res., 54, 37–41.
Vlasova, I. I., Arnhold, J., Osipov, A. N., and Panasenko, O. M. (2006) pH-dependent regulation of myeloperoxi-dase activity, Biochemistry (Moscow), 71, 667–677.
Funding
Funding The authors’ studies cited in this review were supported by the Russian Foundation for Basic Research (projects 17-04-00530 and 18-515-00004) and Russian Science Foundation (project 17-75-30064).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Conflict of interest. The authors declare that there is no conflict of interest.
Russian Text © The Author(s), 2020, published in Uspekhi Biologicheskoi Khimii, 2020, Vol. 60, pp. 75-122.
Rights and permissions
About this article
Cite this article
Panasenko, O.M., Torkhovskaya, T.I., Gorudko, I.V. et al. The Role of Halogenative Stress in Atherogenic Modification of Low-Density Lipoproteins. Biochemistry Moscow 85 (Suppl 1), 34–55 (2020). https://doi.org/10.1134/S0006297920140035
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920140035