Abstract
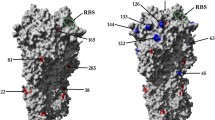
It was previously shown that hemagglutinin residues Thrl55, Glul58, and Ser228 are crucial for the recognition of Neu5Gc. In this study, we demonstrated that the ability to bind the Neu5Gc-terminated receptor is related to the amino acid 145: viruses of years 1972–1999 with Lysl45bindto the receptor, whereas viruses with Asnl 45 do not. Sporadic appearance and disappearance of the ability to bind Neu5Gc oligosaccharides and the absence of Neu5Gc in the composition of human glycoconjugates indicate the non-adaptive nature of this ability. It was previously shown that unlike H1N1 viruses, H3N2 viruses of years 1968–1989 did not distinguish between Neu5Acα2-6Galβ1-4Glc (6′SL) and Neu5Acα2-6Galβ1-4GlcNAc (6′SLN). H3N2 viruses isolated after 1993 have acquired the ability to distinguish between 6’SL and 6′SLN, similarly to H1N1 viruses. We found that the affinity for 6′SLN has gradually increased from 1992 to 2003. After 2003, the viruses lost the ability to bind a number of sialosides, including 6′SL, that were good receptors for earlier H3N2 viruses, and retained high affinity for 6′SLN only, which correlated with the acquisition of new glycosylation sites at positions 122, 133, and 144, as well as Glul90Asp and Gly225Asp substitutions, in hemagglutinin. These substitutions are also responsible for the receptor-binding phenotype of human H1N1 viruses. We conclude that the convergent evolution of the receptor specificity of the H1N1 and H3N2 viruses indicates that 6’SLN is the optimal natural human receptor for influenza viruses.
Similar content being viewed by others
Abbreviations
- CE:
-
embryonated chicken egg
- Glyc-PAA:
-
glycosylated polyacrylamide
- Glyc-PAA-biotin:
-
biotinylated glycosylated polyacrylamide
- HA:
-
hemagglutinin
- NA:
-
neuraminidase
- RBS:
-
receptor-binding site
- Sia:
-
sialic acid (either Neu5Ac or Neu5Gc)
References
Webster, R. G., Bean, W. J., Gorman, O. T., Chambers, T M., and Kawaoka, Y. (1992) Evolution and ecology of influenza A viruses, Microbiol. Rev., 56, 152–179.
Paulson, J. C. (1985) Interactions of animal viruses with cell surface receptors, in The Receptors (Conn, M., ed.) Academic Press, Orlando, FL, Vol. 2, pp. 131–219.
Wiley, D. C, and Skehel, J. J. (1987) The structure and function of the hemagglutinin membrane glycoprotein of influenza virus, Ann. Rev. Biochem., 56, 365–394; doi: https://doi.org/10.1146/annurev.bi.56.070187.002053.
Rogers, G. N., and D’Souza, B. L. (1989) Receptor-binding properties of human and animal HI influenza virus isolates, Virology, 173, 317–322.
Connor, R. J., Kawaoka, Y, Webster, R. G., and Paulson, J. C. (1994) Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates, Virology, 205, 17–23; doi: https://doi.org/10.1006/viro.l994.1615.
Gambaryan, A. S., Tuzikov, A. B., Piskarev, V. E., Yamnikova, S. S., Lvov, D. K., Robertson, J. S., Bovin, N. V., and Matrosovich, M. N. (1997) Specification of receptor-binding phenotypes of influenza virus isolates from different hosts using synthetic sialylglycopolymers: non-egg-adapted human HI and H3 influenza A and influenza B viruses share a common high binding affinity for 6’-sialyl-N-(acetyllactosamine), Virology, 232, 345–350; doi: https://doi.org/10.1006/viro.1997.8572.
Matrosovich, M. N., Gambaryan, A. S., Teneberg, S., Piskarev, V. E., Yamnikova, S. S., Lvov, D. K., Robertson, J. S., and Karlsson, K. A. (1997) Avian influenza A viruses differ from human viruses by recognition of sialyloligosaccharides and gangliosides and by a higher conservation of the HA receptor-binding site, Virology, 233, 224–234; doi: https://doi.org/10.1006/viro.1997.8580.
Matrosovich, M., Tuzikov, A., Bovin, N., Gambaryan, A., Klimov, A., Castrucci, M. R., Donatelli, I., and Kawaoka, Y (2000) Early alterations of the receptor-binding properties of HI, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals, J. Virol., 74, 8502–8512; doi: https://doi.org/10.1128/JVI.74.18.8502-8512.
Gambaryan, A. S., Piskarev, V. E., Yamskov, I. A., Sakharov, A. M., Tuzikov, A. B., Bovin, N., Nifant’ev, N. E., and Matrosovich, M. N. (1995) Human influenza virus recognition of sialyloligosaccharides, FEES Lett., 366, 57–60.
Gambaryan, A. S., Robertson, J. S., and Matrosovich, M. N. (1999) Effects of egg-adaptation on the receptor-binding properties of human influenza A and B viruses, Virology, 258, 232–239; doi: https://doi.org/10.1006/viro.1999.9732.
Grassauer, A, Egorov, A. Y, Ferko, B., Romanova, J., Katinger, H, and Muster, T (1998) A host restriction-based selection system for influenza haemagglutinin trans-fectant viruses, J. Gen. Virol, 79, 1405–1409; doi: https://doi.org/10.1099/0022-1317-79-6-1405.
Nobusawa, E., Ishihara, H, Morishita, T, Sato, K, and Nakajima, K. (2000) Change in receptor-binding specificity of recent human influenza A viruses (H3N2): a single amino acid change in hemagglutinin altered its recognition of sialyloligosaccharides, Virology, 278, 587–596; doi: https://doi.org/10.1006/viro.2000.0679.
Medeiros, R., Escriou, N., Naffakh, N., Manuguerra, J. C., and van der Werf, S. (2001) Hemagglutinin residues of recent human A (H3N2) influenza viruses that contribute to the inability to agglutinate chicken erythrocytes, Virology, 289, 74–85; doi: https://doi.org/10.1006/viro.2001.1121.
Fitch, W M., Bush, R. M., Bender, C. A., and Cox, N. J. (1997) Long term trends in the evolution of H3HA1 human influenza type A, Proc. Natl. Acad. Sci. USA, 94, 7712–7718; doi: https://doi.org/10.1073/pnas.94.15.7712.
Bush, R. M., Bender, C. A, Subbarao, K, Cox, N. L, and Fitch, W M. (1999) Predicting the evolution of human influenza A, Science, 286, 1921–1925.
Wang, X., Ilyushina, N. A., Lugovtsev, V. Y, Bovin, N.V, Couzens, L., Gao, J., Donnelly, R. P., Eichelberger, M. C, and Wan, H. (2017) Amino acids in hemagglutinin antigenic site B determine antigenic and receptor binding differences between A(H3N2)v and ancestral seasonal H3N2 influenza viruses, J. Virol., 91, e01512–01516; doi: https://doi.org/10.1128/JVI.01512-16.
Mochalova, L., Gambaryan, A., Romanova, J., Tuzikov, A., Chinarev, A., Katinger, D., Katinger, H, Egorov, A., and Bovin, N. (2003) Receptor-binding properties of modern human influenza viruses primarily isolated in Vero and MDCK cells and chicken embryonated eggs, Virology, 313, 473–480.
Stevens, J., Chen, L. M., Carney, P. J., Garten, R., Foust, A., Le, J., Pokorny, B. A., Manojkumar, R., Silverman, J., Devis, R, Rhea, K, Xu, X, Bucher, D. L, Paulson, J. C, Cox, N. J., Klimov, A, and Donis, R O. (2010) Receptor specificity of influenza A H3N2 viruses isolated in mammalian cells and embryonated chicken eggs, J. Virol., 84, 8287–8299; doi: https://doi.org/10.1128/JVI.00058-10.
Wu, N. C, Xie, J., Zheng, T., Nycholat, C. M., Grande, G., Paulson, J. C, Lerner, R. A., and Wilson, I. A. (2017) Diversity of functionally permissive sequences in the receptor-binding site of influenza hemagglutinin, Cell Host Microbe, 21, 742–753; doi: https://doi.org/10.1016/j.chom.2017.05.011.
Gulati, S., Smith, D. E, Cummings, R. D., Couch, R. B., Griesemer, S. B., St George, K., Webster, R. G., and Air, G. M. (2013) Human H3N2 influenza viruses isolated from 1968 to 2012 show varying preference for receptor substructures with no apparent consequences for disease or spread, PLoSOne, 8, 66325; doi: https://doi.org/10.1371/journal.pone.0066325.
Yang, H., Carney, P. J., Chang, J. C., Guo, Z., Villanueva, J. M., and Stevens, J. (2015) Structure and receptor binding preferences of recombinant human A(H3N2) virus hemagglutinins, Virology, 477, 18–31; doi: https://doi.org/10.1016/j.virol.2014.12.024.
Peng, W, de Vries, R. P., Grant, O. C, Thompson, A. J., McBride, R, Tsogtbaatar, B., Lee, P. S., Razi, N., Wilson, I. A, Woods, R. J., and Paulson, J. C. (2017) Recent H3N2 viruses have evolved specificity for extended, branched human-type receptors, conferring potential for increased avidity, Cell Host Microbe, 21, 23–34; doi: https://doi.org/10.1016/j.chom.2016.11.004.
Bovin, N. V., Korchagina, E. Yu., Zemlyanukhina, T. V, Byramova, N. E., Galanina, O. E., Zemlyakov, A. E., Ivanov, A. E., Zubov, V. P., and Mochalova, L. V. (1993) Synthesis of polymeric neoglycoconjugates based on N-substituted polyacrylamides, Glycoconj. J., 10, 142–151.
Shilova, N. V., Galanina, O. E., Pochechueva, T. V., Chinarev, A. A., Kadykov, V. A., Tuzikov, A. B., and Bovin, N. V. (2005) High molecular weight neoglycoconjugates for solid phase assays, Glycoconj. J., 22, 43–51; doi: https://doi.org/10.1007/s10719-005-0280-y.
Byramova, N. E., Tuzikov, A. B., and Bovin, N. V. (1992) A simple procedure for the synthesis of the methyl and benzyl glycosides of Neu5Ac and 4-epi-Neu5Ac, Carbohydr. Res., 237, 161–175; doi: https://doi.org/10.1016/S0008-6215(92)84240-S.
Matrosovich, M. N., Mochalova, L. V, Marinina, V. P., Byramova, N. E., and Bovin, N. V. (1990) Synthetic polymeric sialoside inhibitors of influenza virus receptor-binding activity, FEES Lett., 272, 209–212.
Gambaryan, AS., and Matrosovich, M. N. (1992) A solid-phase enzyme-linked assay for influenza virus receptor-binding activity, J. Virol. Methods, 39, 111–123.
Hayase, T, Rice, K. G., Dziegielewska, K. M., Kuhlenschmidt, M., Reilly T., and Lee, Y. C. (1992) Comparison of N-glycosides of fetuins from different species and human alpha 2-HS-glycoprotein, Biochemistry, 31, 4915–4921.
Anders, E. M., Scalzo, A. A., Rogers, G. N., and White, D. O. (1986) Relationship between mitogenic activity of influenza viruses and the receptor-binding specificity of their hemagglutinin molecules, J. Virol., 60, 476–482.
Suzuki, Y (2001) Host mediated variation and receptor binding specificity of influenza viruses, Adv. Exp. Med. Biol, 491, 445–451.
Matrosovich, M. N., Gambaryan, A. S., Tuzikov, A. B., Byramova, N. E., Mochalova, L. V., Golbraikh, A. A., Shenderovich, M. D., Finne, J., and Bovin, N. V. (1993) Probing of the receptor-binding sites of the HI and H3 influenza A and influenza B virus hemagglutinins by synthetic and natural sialosides, Virology, 196, 111–121; doi: https://doi.org/10.1006/viro.1993.1459.
Pritchett, T. J., Brossmer, R., Rose, U., and Paulson, J. C. (1987) Recognition of monovalent sialosides by influenza virus H3 hemagglutinin, Virology, 160, 502–506.
Eisen, M. B., Sabesan, S., Skehel, J. J., and Wiley D. C. (1997) Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin-sialyloligosaccharide complexes determined by X-ray crystallography, Virology, 232, 19–31; doi: https://doi.org/10.1006/viro.1997.8526.
Gamblin, S. J., Haire, L. E., Russell, R. J., Stevens, D. J., Xiao, B., Ha, Y., Vasisht, N., Steinhauer, D. A., Daniels, R. S., Elliot, A, Wiley D. C., and Skehel, J. J. (2004) The structure and receptor-binding properties of the 1918 influenza hemagglutinin, Science, 303, 1838–1842; doi: https://doi.org/10.1126/science.1093155.
Acknowledgements
We regret to inform that our colleague Alexander Klimov has died.
Funding
This study was supported by the Russian Foundation for Basic Research (projects 02-04-48109, 01-04-49300, 14-04-00547, and 17-04-00148) and the International Science and Technology Center (grant no. 5-2464).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare no conflict of interest in financial or any other sphere.
Ethical approval. This article does not contain any studies with animals or human participants performed by any of the authors.
Additional information
Published in Russian in Biokhimiya, 2019, Vol. 84, No. 10, pp. 1450–1459.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM19-109, August 26, 2019.
Rights and permissions
About this article
Cite this article
Gambaryan, A.S., Balish, A., Klimov, A.I. et al. Changes in the Receptor-Binding Properties of H3N2 Viruses during Long-Term Circulation in Humans. Biochemistry Moscow 84, 1177–1185 (2019). https://doi.org/10.1134/S0006297919100067
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297919100067