Abstract
Glycogen is a strongly branched polymer of α-D-glucose, with glucose residues in the linear chains linked by 1→4-bonds (~93% of the total number of bonds) and with branching after every 4-8 residues formed by 1→6-glycosidic bonds (~7% of the total number of bonds). It is thought currently that a fully formed glycogen molecule (β-particle) with the self-glycosylating protein glycogenin in the center has a spherical shape with diameter of ~42 nm and contains ~ 55,000 glucose residues. The glycogen molecule also includes numerous proteins involved in its synthesis and degradation, as well as proteins performing a carcass function. However, the type and force of bonds connecting these proteins to the polysaccharide moiety of glycogen are significantly different. This review presents the available data on the spatial structure of the glycogen molecule and its changes under various physiological and pathological conditions.
Similar content being viewed by others
Abbreviations
- BE:
-
branching enzyme
- GDE:
-
glycogen debranching enzyme
- GP:
-
glycogen phosphorylase
- G-1-P:
-
glu-cose-1-phosphate
- G-6-P:
-
glucose-6-phosphate
- GPa:
-
active form of glycogen phosphorylase
- GS:
-
glycogen synthase
- MG:
-
macroglycogen
- PG:
-
proglycogen
References
Schmidt-Nielsen, K. (1982) Animal Physiology. Adaptation and Environment [Russian translation], Mir, Moscow.
Danovaro, R., Dell’Anno, A., Pusceddu, A., Gambi, C., Heiner, I., and Kristensen, R. M. (2010) The first meta-zoan living in permanently anoxic conditions, BMC Biol., 8, 30.
Kasting, J. F., and Siefert, J. (2002) Life and the evolution of Earth’s atmosphere, Science, 296, 1066–1068.
Knoll, A. H. (2003) Life on a Young Planet: The First Tree Billion Years of Evolution on Earth, Princeton, Princeton University Press, Chichester.
Holand, H. D. (2006) The oxygenation of the atmosphere and oceans, Phil. Trans. R. Soc. B, 361, 903–915.
Ball, S., Colleoni, C., Cenci, U., Raj, J. N., and Tirtiaux, C. (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the estab-lishment of plastid endosymbiosis, J. Exp. Botany, 62, 1775–1801.
Bernard, C. (1857) Nouvelles recherches experimentales sur les phenomenes glycogeniques du foie, Mum. Soc. Biol., 4, 1–7.
Harris, R. A. (1992) Textbook of Biochemistry with Clinical Correlations (Davlin, T. M., ed.) Wiley Liss, N. Y., pp. 291–358.
Ferrer, J. C., Favre, C., Gomis, R. R., Fernandez-Novell, J. M., Garcia-Rocha, M., de la Iglesia, N., Cid, E., and Guinovart, J. J. (2003) Control of glycogen deposition, FEBS Lett., 546, 27–132.
Greenberg, C. C., Jurczak, M. J., Danos, A. M., and Brady, M. J. (2006) Glycogen branches out: new perspec-tives on the role of glycogen metabolism in the integration of metabolic pathways, Am. J. Physiol. Endocrinol. Metab., 291, E1–E8.
Jurczak, M. J., Danos, A. M., Rehrmann, V. R., and Brady, M. J. (2008) The role of protein translocation in the regu-lation of metabolism, J. Cell Biochem., 104, 435–443.
Jungermann, K. (1992) Role of intralobular compartmen-tation in hepatic metabolism, Diabete Metab., 18, 81–86.
Rajvanshi, P., Liu, D., Ott, M., Gagandeep, S., Schilsky, M. L., and Gupta, S. (1998) Fractionation of rat hepato-cyte subpopulations with varying metabolic potential, pro-liferative capacity, and retroviral gene transfer efficiency, Exp. Cell. Res., 244, 405–419.
Teutsch, H. F., Schuerfeld, D., and Groezinger, E. (1999) Three-dimensional reconstruction of parenchymal units in the liver of the rat, Hepatology, 29, 494–505.
Kudryavtseva, M. V., Bezborodkina, N. N., Radchenko, V. G., Okovity, S. V., and Kydryavtsev, B. N. (2001) Metabolic heterogeneity of glycogen in hepatocytes of patients with liver cirrhosis: the glycogen of the liver lobule zones in cir-rhosis, Europ. J. Gastroenterol. Hepatol., 13, 693–697.
Roach, P. J., Depaoli-Roach, A. A., Hurley, T. D., and Tagliabracci, V. S. (2012) Glycogen and its metabolism: some new development and old themes, Biochem. J., 441, 763–787.
Shearer, J., and Graham, T. E. (2004) Novel aspects of skeletal muscle glycogen and its regulation during rest and exercise, Exerc. Sport. Sci. Rev., 32, 120–126.
Roach, P. J. (2002) Glycogen and its metabolism, Curr. Mol. Med., 2, 101–120.
Minassian, B. A., Lee, J. R., Herbrick, J. A., Huizenga, J., Soder, S., Mungall, A. J., Dunham, I., Gardner, R., Fong, C. Y., Carpenter, S., Jardim, L., Satishchandra, P., Andermann, E., Snead, O. C., 3rd, Lopes-Cendes, I., Tsui, L. C., Delgado-Escueta, A. V., Rouleau, G. A., and Scherer, S. W. (1998) Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy, Nat. Genet., 20, 171–174.
Ganesh, S., Amano, K., Delgado-Escueta, A. V., and Yamakawa, K. (1999) Isolation and characterization of mouse homologue for the human epilepsy gene, Biochem. Biophys. Res. Commun., 257, 24–28.
Jiang, S., Heller, B., Tagliabracci, V. S., Zhai, L., Irimia, J. M., Depaoli-Roach, A. A., Wells, C. D., Skurat, A. V., and Roach, P. J. (2010) Starch binding domain-containing pro-tein 1/genethonin 1 is a novel participant in glycogen metabolism, J. Biol. Chem., 285, 34960–34971.
Stapleton, D., Nelson, C., Parsawar, K., McClain, D., Gilbert-Wilson, R., Barker, E., Rudd, B., Brown, K., Hendrix, W., O’Donnell, P., and Parker, G. (2010) Analysis of hepatic glycogen-associated proteins, Proteomics, 10, 2320–2329.
Caudwell, F. B., and Cohen, P. (1980) Purification and subunit structure of glycogen-branching enzyme from rab-bit skeletal muscle, Eur. J. Biochem., 109, 391–394.
Krisman, C. R., and Barengo, R. A. (1975) A precursor of glycogen biosynthesis: alpha-1,4-glucan-protein, Eur. J. Biochem., 52, 117–123.
Alonso, M. D., Lomako, J., Lomako, W. M., and Whelan, W. J. (1995) A new look at the biogenesis of glycogen, FASEB J., 9, 1126–1137.
Cao, Y., Mahrenholz, A. M., DePaoli-Roach, A. A., and Roach, P. J. (1993) Characterization of rabbit skeletal mus-cle glycogenin: tyrosine 194 is essential for function, J. Biol. Chem., 268, 14687–14693.
Hurley, T. D., Walls, C., Bennett, J. R., Roach, P. J., and Wang, M. (2006) Direct detection of glycogenin reaction products during glycogen initiation, Biochem. Biophys. Res. Commun., 348, 374–378.
Roach, P. J., and Skurat, A. V. (1997) Self-glucosylating initiator proteins and their role in glycogen biosynthesis, Prog. Nucleic Acid Res. Mol. Biol., 57, 289–316.
Lomako, J., Lomako, W. M., and Whelan, W. J. (2004) Glycogenin: the primer for mammalian and yeast glycogen synthesis, Biochim. Biophys. Acta, 1673, 45–55.
Fernandez-Novell, J. M., Lopez-Iglesias, C., Ferrer, J. C., and Guinovart, J. J. (2002) Zonal distribution of glycogen syn-thesis in isolated rat hepatocytes, FEBS Lett., 531, 222–228.
Larner, J. (1955) Branching enzyme from liver, Methods Enzymol., 1, 222–225.
Krisman, C. R. (1962) α-l,4-Glucan: α-l,4-glucan 6-glyco-syltransferase from liver, Biochim. Biophys. Acta, 65, 307–315.
Brown, B. I., and Brown, D. H. (1966) α-l,4-Glucan 6-glycosyltransferase from mammalian muscle, Methods Enzymol., 8, 395–403.
Melendez, R., Melendez-Hevia, E., and Cascante, M. (1997) How did glycogen structure evolve to satisfy the requirement for rapid mobilization of glucose? A problem of physical constraints in structure building, J. Mol. Evol., 45, 446–455.
Bezborodkina, N. N., Okovityi, S. V., and Kudryavtsev, B. N. (2008) Carbohydrate Metabolism in Chronic Damages of the Liver [in Russian], Sintez-Buk, St. Petersburg.
Biorn, A. C., and Graves, D. J. (2001) The amino-terminal tail of glycogen phosphorylase is a switch for controlling phosphorylase conformation, activation, and response to ligands, Biochemistry, 40, 5181–5189.
Newsholm, E., and Start, C. (1977) Regulation in Metabolism [Russian translation], Mir, Moscow.
Goldsmith, E., Sprang, S., and Fletterick, R. (1982) Structure of maltoheptaose by difference Fourier methods and a model for glycogen, J. Mol. Biol., 156, 411–427.
Geddes, R., Jeyarathan, P., and Taylor, J. A. (1992) Molecular and metabolic aspects of lysosomal glycogen, Carbohydr. Res., 227, 339–349.
Geddes, R. (1985) The Polysaccharides, Vol. 3 (Aspinall, G. O., ed.), Academic Press, Orlando, pp. 283–336.
Stepanenko, B. N., and Bobrova, L. N. (1974) Modern concepts about micro-and macromolecular structure of glycogen, Usp. Biol. Khim., 15, 195–207.
Geddes, R., and Stratton, G. C. (1977) The influence of lysosomes on glycogen metabolism, Biochem. J., 163, 193–200.
Wanson, J. C., and Drochmans, P. (1968) Rabbit skeletal muscle glycogen. A morphological and biochemical study of glycogen β-particles isolated by the precipitation-cen-trifugation method, J. Cell Biol., 38, 130–150.
Devos, P., Baudhuin, P., Van Hoof, F., and Hers, H. G. (1983) The alpha particulate liver glycogen. A morphomet-ric approach to the kinetics of its synthesis and degradation, Biochem. J., 209, 159–165.
Rybicka, K. K. (1996) Glycosomes–the organelles of glycogen metabolism, Tissue Cell, 28, 253–265.
Sullivan, M. A., Vilaplana, F., Cave, R. A., Stapleton, D., Gray-Weale, A. A., and Gilbert, R. G. (2010) Nature of α-and β-particles in glycogen using molecular size distribu-tions, Biomacromolecules, 11, 1094–1100.
Besford, Q. A., Sullivan, M. A., Zheng, L., Gilbert, R. G., Stapleton, D., and Gray Weale, A. (2012) The structure of cardiac glycogen in healthy mice, Int. J. Biol. Macromol., 51, 887–891.
Devos, P., and Hers, H. G. (1979) A molecular order in the synthesis and degradation of glycogen in the liver, Eur. J. Biochem., 99, 161–167.
Devos, P., and Hers, H. G. (1980) Glycogen in rat adipose tissue: sequential synthesis and random degradation, Biochem. Biophys. Res. Commun., 95, 1031–1036.
Orrell, S. A., and Bueding, E. (1964) A comparison of products obtained by various procedures used for the extraction of glycogen, J. Biol. Chem., 239, 4021–4026.
Nakamura, M. (1977) Handbook of Starch Science, Asakura-Shoten, Tokyo.
Hata, K., Hata, M., Hata, M., and Matsuda, K. (1984) A proposed model of glycogen particle, J. Jpn. Soc. Starch. Sci., 31, 146–155.
Sullivan, M. A., Aroney, S. T., Li, S., Warren, F. J., Joo, J. S., Mak, K. S., Stapleton, D. I., Bell-Anderson, K. S., and Gilbert, R. G. (2014) Molecular insights into glycogen α-particle formation, Biomacromolecules, 15, 660–665.
Stetten, M. R., and Stetten, D., Jr. (1958) Influence of molecular size of glycogen on the phosphorylase reaction, J. Biol. Chem., 232, 489–504.
Melendez-Hevia, E., Waddell, T. G., and Shelton, E. D. (1993) Optimization of molecular design in the evolution of metabolism: the glycogen molecule, Biochem. J., 295 (Pt. 2), 477–483.
Meyer, R. H., and Bernfeld, P. (1940) Recherches sur l’amidon V. L’amilopectine, Helv. Chim. Acta, 23, 875–885.
Whelan, W. J. (1971) Enzymic explorations of the struc-tures of starch and glycogen, Biochem. J., 122, 609–622.
Walker, G. J., and Whelan, W. J. (1960) The mechanism of carbohydrase action. 8. Structures of the muscle-phospho-rylase limit dextrins of glycogen and amylopectin, Biochem. J., 76, 264–270.
Shearer, J., and Graham, T. E. (2002) New perspectives on the storage and organization of muscle glycogen, J. Appl. Physiol., 27, 179–203.
Graham, T. E., Yuan, Z., Hill, A. K., and Wilson, R. J. (2010) The regulation of muscle glycogen: the granule and its proteins, Acta Physiol., 199, 489–498.
Madsen, N. B., and Cori, C. F. (1958) The binding of glycogen and phosphorylase, J. Biol. Chem., 233, 1251–1256.
Melendez, R., Melendez-Hevia, E., Mas, F., Mach, J., and Cascante, M. (1998) The fractal structure of glycogen: a clever solution to optimize cell metabolism, Biophys. J., 75, 106–114.
Melendez-Hevia, E., Waddel, T. G., Raposo, R. R., and Lupianez, J. A. (1995) Evolution of metabolism: optimiza-tion of glycogen structure, J. Biol. Systems, 3, 177–186.
Geddes, R. (1986) Glycogen: a metabolic viewpoint, Bioscience Rep., 6, 415–428.
Marshall, J. J. (1974) Application of enzymic methods to the structural analysis of polysaccharides, Adv. Carbohydr. Chem. Biochem., 30, 257–370.
Gunja-Smith, Z., Marshall, J. J., Mercier, C., Smith, E. E., and Whelan, W. J. (1970) A revision of the Meyer–Bernfeld model of glycogen and amylopectin, FEBS Lett., 12, 101–104.
Gessler, K., Uson, I., Takaha, T., Krauss, N., Smith, S. M., Okada, S., Sheldrick, G. M., and Saenger, W. (1999) V-amylose at atomic resolution: X-ray structure of a cycloamylose with 26 glucose residues (cyclomaltohexa-icosaose), Proc. Natl. Acad. Sci. USA, 96, 4246–4251.
Mercier, C., and Whelan, W. J. (1970) The fine structure of glycogen from type IV glycogen storage disease, Eur. J. Biochem., 16, 579–583.
Garyaly, P., Segvich, D. M., DePaoli-Roach, A. A., and Roach, P. J. (2014) Protein degradation and quality control in cells from laforin and malin knockout mice, J. Biol. Chem., 289, 20606–20614.
Jiang, S., Wells, C. D., and Roach, P. J. (2011) Starch-binding domain-containing protein 1 (Stbd1) and glycogen metabolism: identification of the Atg8 family interacting motif (AIM) in Stbd1 required for interaction with GABARAPL1, Biochem. Biophys. Res. Commun., 413, 420–425.
Sun, T., Yi, H., Yang, C., Kishnani, P. S., and Sun, B. (2016) Starch binding domain-containing protein 1 plays a dominant role in glycogen transport to lysosomes in liver, J. Biol. Chem., 291, 16479–16484.
Willstatter, R., and Rhodewald, M. (1934) Protein binding of physiologically important substances. The conditions of glycogen in liver, muscle and leucocytes, Z. Physiol. Chem., 225, 103–124.
Bloom, W. L., Lewis, G. T., Schumpert, M. Z., and Tsung-men Shen (1951) Glycogen fractions of liver and muscle, J. Biol. Chem., 188, 631–636.
Russel, J. A., and Bloom, W. L. (1955) Extractable and residual glycogen in tissues of the rat, Am. J. Physiol., 183, 345–355.
Weisberg, J., and Rodbard, S. (1958) Distribution of glyco-gen in the rat heart, Am. J. Physiol., 193, 466–468.
Kugler, J. H., and Wilkinson, W. J. (1959) A relation between total glycogen content of ox myocardium and its histochemical demonstration, J. Histochem. Cytochem., 7, 298–402.
Kugler, J. H., and Wilkinson, W. J. (1960) Glycogen frac-tions and their role in the histochemical detection of glyco-gen, J. Histochem. Cytochem., 8, 195–199.
Kugler, J. H., and Wilkinson, W. J. (1961) The basis for the histochemical detection of glycogen, J. Histochem. Cytochem., 9, 498–503.
Kudryavtseva, M. V., Kudryavtsev, B. N., and Rozanov, Y. M. (1974) About two fractions of glycogen in the cells of rat liver (cytofluorimetric study), Tsitologiya, 16, 851–858.
Prins, D. A., and Jeanlos, R. W. (1948) Chemistry of the carbohydrates, Annu. Rev. Biochem., 17, 67–96.
Kits Van Heijningen, A. J., and Kemp, A. (1955) Free and fixed glycogen in rat muscle, Biochem. J., 59, 487–491.
Rozenfeld, E. L., and Popova, I. A. (1979) Glycogen Disease [in Russian], Meditsina, Moscow.
Lomako, J., Lomako, W. M., and Whelan, W. J. (1991) Proglycogen: a low-molecular-weight of muscle glycogen, FEBS Lett., 279, 223–228.
Lomako, J., Lomako, W. M., Whelan, W. J., Dombro, R. S., Neary, J. T., and Norenberg, M. D. (1993) Glycogen synthesis in the astrocyte: from glycogenin to proglycogen to glycogen, FASEB J., 7, 1386–1393.
Shearer, J., Graham, T. E., Battram, D. S., Robinson, D. L., Richter, E. A., Wilson, R. J., and Bakovic, M. (2005) Glycogenin activity and mRNA expression in response to volitional exhaustion in human skeletal muscle, J. Appl. Physiol., 99, 957–962.
Kraniou, Y., Cameron-Smith, D., Misso, M., Collier, G., and Hargreaves, M. (2000) Effects of exercise on GLUT-4 and glycogenin gene expression in human skeletal muscle, J. Appl. Physiol., 88, 794–796.
Arkinstall, M. J., Bruce, C. R., Clark, S. A., Rickards, C. A., Burke, L. M., and Hawley, J. A. (2004) Regulation of fuel metabolism by pre-exercise muscle glycogen content and exercise intensity, J. Appl. Physiol., 97, 2275–2283.
Marchand, I., Tarnopolsky, M., Adamo, K. B., Bourgeois, J. M., Chorneyko, K., and Graham, T. E. (2007) Quantitative assessment of human muscle glycogen granules size and number in subcellular locations during recovery from prolonged exercise, J. Physiol., 580 (Pt. 2), 617–628.
Adamo, K. B., and Graham, T. E. (1998) Comparison of traditional measurement with macroglycogen and pro-glycogen analysis of muscle glycogen, J. Appl. Physiol., 84, 908–913.
Battram, D. S., Shearer, J., Robinson, D., and Graham, T. E. (2004) Caffeine ingestion does not impede the resyn-thesis of proglycogen and macroglycogen after prolonged exercise and carbohydrate supplementation in humans, J. Appl. Physiol., 96, 943–950.
Wilson, R. J. (2009) Relating Glycogenin Protein Levels and Glycogen Content Post-contraction in Human and Rodent Skeletal Muscle, Thesis University Guelph.
Granlund, A., Jensen-Waern, M., and Essen-Gustavsson, B. (2011) The influence of the PRKAG3 mutation on glycogen, enzyme activities and fibre types in different skeletal muscles of exercise trained pigs, Acta Vet. Scand., 53, 20.
Graham, T. E., Adamo, K. B., Shearer, J., Marchand, I., and Saltin, B. (2001) Pro-and macroglycogenolysis: rela-tionship with exercise intensity and duration, J. Appl. Physiol., 90, 873–879.
James, A. P., Barnes, P. D., Palmer, T. N., and Fournier, P. A. (2008) Proglycogen and macroglycogen: artifacts of glycogen extraction? Metabolism, 57, 535–543.
Kudryavtseva, M. V., and Shalakhmetova, T. M. (1976) Microfluorimetrical study of glycogen fractions in the cells of rat liver under different conditions of liver perfusion and animal nutrition, Tsitologiya, 18, 506–509.
Kudryavtseva, M. V., Skorina, A. D., and Kudryavtsev, B. N. (1988) Cytofluorimetrical study of glycogen fractions in cells of the liver from patients with chronic viral and alco-hol hepatitis, Tsitologiya, 30, 705–709.
Kudryavtseva, M. V., Emel’yanov, A. V., Sakuta, G. A., Skorina, A. D., Sleptsova, L. A., and Kudryavtsev, B. N. (1992) Cytofluorimetrical study of glycogen content and its fractions in hepatocytes of patients with liver cirrhosis of different etiology, Tsitologiya, 34, 100–107.
Kudryavtseva, M. V., Bezborodkina, N. N., Radchenko, V. G., Okovity, S. V., Ivanova, O. V., and Kudryavtsev, B. N. (2000) Metabolic heterogeneity of glycogen in hepato-cytes of patients with liver cirrhosis, Tsitologiya, 42, 550–554.
Kudryavtseva, M. V., Bezborodkina, N. N., Okovity, S. V., and Kudryavtsev, B. N. (2002) Study on the influence of bemithyl on the carbohydrate metabolism of the cirrhoti-cally-altered rat liver, Tsitologiya, 44, 166–174.
Kudryavtseva, M. V., Bezborodkina, N. N., Okovity, S. V., and Kudryavtsev, B. N. (2003) Effects of the 2-ethyl-thiobenzimidazole hydrobromide (bemithyl) on carbohy-drate metabolism in cirrhotic rat liver, Exp. Toxic. Pathol., 54, 339–347.
Kudryavtseva, M. V., and Shalakhmetova, T. M. (1979) Cytofluorimetric study of glycogen contents and its frac-tions in the cells of rat liver during the 1st week of post-natal ontogenesis, Tsitologiya, 21, 566–571.
Kudryavtseva, M. V., and Zavadskaya, E. E. (1982) Cytofluorimetric study of glycogen contents and its frac-tions in the cells of rat liver under conditions of its synthe-sis and degradation, Tsitologiya, 24, 777–783.
Kudryavtseva, M. V., Sakuta, G. A., Stein, G. I., and Kudryavtsev, B. N. (1990) Dynamics of glycogen synthesis in hepatocytes from different lobes of the rat liver, Tsitologiya, 32, 1010–1018.
Kudryavtseva, M. V., Emel’yanov, A. V., Sakuta, G. A., Bezborodkina, N. N., and Kudryavtsev, B. N. (1998) Rehabilitation of the glycogen-generating function of hepatocytes of the cirrhotic rat liver on the high-carbohy-drate diet, Tsitologiya, 40, 133–142.
Chestnova, A. Y. (2016) The Content and Structure of Glycogen in Hepatocytes of Human and Rat Normal and Cirrhotic Liver, Candidates’ dissertation (in Biology) [in Russian], Institute of Cytology, Russian Academy of Sciences, St. Petersburg.
Ghosh, P., Heath, A. C., Donahue, M. J., and Masaracchia, R. A. (1989) Glycogen synthesis in the obliquely striated muscle of Ascaris suum, Eur. J. Biochem., 183, 679–685.
Farkas, I., Hardy, T. A., Goebl, M. G., and Roach, P. J. (1991) Two glycogen synthase isoforms in Saccharomyces cerevisiae are coded by distinct genes that are differential-ly controlled, J. Biol. Chem., 266, 15602–15607.
Sjostrom, M., Friden, J., and Ekblom, B. (1982) Fine structural details of human muscle fibres after fibre type specific glycogen depletion, Histochemistry, 76, 425–438.
Elsner, P., Quistorff, B., Hansen, G. H., and Grunnet, N. (2002) Partly ordered synthesis and degradation of glyco-gen in cultured rat myotubes, J. Biol. Chem., 277, 4831–4838.
Van der Kleji, B. J. (1951) A rapid determination of glyco-gen in tissues, Biochim. Biophys. Acta, 7, 481–482.
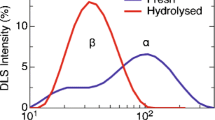
Powell, P. O., Sullivan, M. A., Sheehy, J. J., Schulz, B. L., Warren, F. J., and Gilbert, R. G. (2015) Acid hydrolysis and molecular density of phytoglycogen and liver glycogen helps understand the bonding in glycogen α (composite) particles, PloS One, 10, e0134065.
Stetten, M. R., and Stetten, D., Jr. (1954) A study of the nature of glycogen regeneration in the intact animal, J. Biol. Chem., 207, 331–340.
Roach, P. J., Skurat, A. V., and Harris, R. A. (2001) Handbook of Physiology. Section 7: The Endocrine System. Vol. II. The Endocrine Pancreas and Regulation of Metabolism (Jefferson, L. S., and Cherrington, A. D., eds.), Oxford University Press, Oxford, pp. 609–647.
Prats, C., Helge, J. W., Nordby, P., Qvortrup, K., Ploug, T., Dela, F., and Wojtaszewski, J. F. (2009) Dual regulation of muscle glycogen synthase during exercise by activation and compartmentalization, J. Biol. Chem., 284, 15692–15700.
DiNuzzo, M. (2013) Kinetic analysis of glycogen turnover: relevance to human brain 13C-NMR spectroscopy, J. Cereb. Blood Flow Metab., 33, 1540–1548.
Fessenden, J. D. (2009) Forster resonance energy transfer measurements of ryanodine receptor type 1 structure using a novel site-specific labeling method, PloS One, 4, e7338.
Grecco, H. E., and Verveer, P. J. (2011) FRET in cell biol-ogy: still shining in the age of super-resolution? ChemPhysChem, 12, 484–490.
Miyawaki, A. (2011) Development of probes for cellular functions using fluorescent proteins and fluorescence res-onance energy transfer, Annu. Rev. Biochem., 80, 357–373.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N. N. Bezborodkina, A. Yu. Chestnova, M. L. Vorobev, B. N. Kudryavtsev, 2018, published in Biokhimiya, 2018, Vol. 83, No. 5, pp. 627–644.
Rights and permissions
About this article
Cite this article
Bezborodkina, N.N., Chestnova, A.Y., Vorobev, M.L. et al. Spatial Structure of Glycogen Molecules in Cells. Biochemistry Moscow 83, 467–482 (2018). https://doi.org/10.1134/S0006297918050012
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297918050012