Abstract
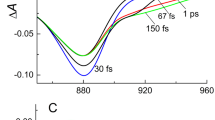
Energy relaxation was studied with difference femtosecond spectroscopy in reaction centers of the YM210L mutant of the purple photosynthetic bacterium Rhodobacter sphaeroides at low temperature (90 K). A dynamical long-wavelength shift of stimulated emission of the excited state of the bacteriochlorophyll dimer P was found, which starts simultaneously with P* formation and is accompanied by a change in the spectral shape of this emission. The characteristic value of this shift was about 30 nm, and the characteristic time about 200 fs. Difference kinetics ΔA measured at fixed wavelengths demonstrate the femtosecond shift of the P* stimulated emission appearing as a dependence of these kinetics on wavelength. We found that the reported long-wavelength shift can be explained in terms of electron-vibrational relaxation of the P* excited state with time constants of vibrational and electronic relaxation of 100 and 50 fs, respectively. Alternative mechanisms of the dynamical shift of the P* stimulated emission spectrum are also discussed in terms of energy redistribution between vibrational modes or coherent excitation of the modes.
Similar content being viewed by others
Abbreviations
- ΔA :
-
absorption change (illumination minus darkness)
- BA and BB :
-
monomeric bacteriochlorophylls in A and B chains, respectively
- HA and HB :
-
bacteriopheophytins in A and B chains, respectively
- P:
-
bacteriochlorophyll dimer
- PA and PB :
-
bacteriochlorophyll molecules that make up P
- QA and QB :
-
primary and secondary quinones, respectively
- RC:
-
reaction center
- Rba. :
-
Rhodobacter
References
Yakovlev, A. G., and Shuvalov, V. A. (2016) Physical stage of charge separation during photosynthesis, Adv. Phys. Sci., 126, 597–625.
Lockhart, D. J., and Boxer, S. G. (1987) Magnitude and direction of the change in dipole moment associated with excitation of the primary electron donor in Rhodopseudomonas sphaeroides reaction centers, Biochemistry, 26, 664–668.
Philipson, K. D., and Sauer, K. (1973) Comparative study of the circular dichroism spectra of reaction centers from several photosynthetic bacteria, Biochemistry, 12, 535–539.
Van Brederode, M. E., and Van Grondelle, R. (1999) New and unexpected routes for ultrafast electron transfer in photosynthetic reaction centers, FEBS Lett., 455, 1–7.
Cherepy, N. J., Shreve, A. P., Moore, L. J., Franzen, S., Boxer, S. G., and Mathies, R. A. (1994) Near-infrared resonance Raman spectroscopy of the special pair and the accessory bacteriochlorophylls in photosynthetic reaction centers, J. Phys. Chem., 98, 6023–6029.
Cherepy, N. J., Shreve, A. P., Moore, L. J., Boxer, S. G., and Mathies, R. A. (1997) Temperature dependence of the Qy resonance Raman spectra of bacteriochlorophylls, the primary electron donor, and bacteriopheophytins in the bacterial photosynthetic reaction center, Biochemistry, 36, 8559–8566.
Cherepy, N. J., Shreve, A. P., Moore, L. J., Boxer, S. G., and Mathies, R. A. (1997) Electronic and nuclear dynamics of the accessory bacteriochlorophylls in bacterial photosynthetic reaction centers from resonance Raman intensities, J. Phys. Chem., 101, 3250–3260.
Klevanik, A. V., Ganago, A. O., Shkuropatov, A. Ya., and Shuvalov, V. A. (1988) Electron-phonon and vibronic structure of absorption spectra of the primary electron donor in reaction centers of Rhodopseudomonas viridis, Rhodobacter sphaeroides and Chloroflexus aurantiacus at 1.7-70K, FEBS Lett., 237, 61–64.
Streltsov, A. M., Vulto, S. I. E., Shkuropatov, A. Ya., Hoff, A. J., Aartsma, T. J., and Shuvalov, V. A. (1998) BA and BB absorbance perturbations induced by coherent nuclear motions in reaction centers from Rhodobacter sphaeroides upon 30-femtosecond excitation of the primary donor, J. Phys. Chem., 102, 7293–7298.
Vos, M. H., Jones, M. R., Hunter, C. N., Breton, J., Lambry, J.-C., and Martin, J.-L. (1994) Coherent dynamics during the primary electron-transfer reaction in membrane-bound reaction centers of Rhodobacter sphaeroides, Biochemistry, 33, 6750–6757.
Yakovlev, A. G., Shkuropatov, A. Ya., and Shuvalov, V. A. (2002) Nuclear wavepacket motion between P* and P+BA − potential surfaces with a subsequent electron transfer to HA in bacterial reaction centers at 90 K. Electron transfer pathway, Biochemistry, 41, 14019–14027.
Shuvalov, V. A., Klevanik, A. V., Ganago, A. O., Shkuropatov, A. Ya., and Gubanov, V. S. (1988) Burning of a narrow spectral hole at 1.7 K in the absorption band of the primary electron donor of Rhodopseudomonas viridis reaction centers with blocked electron transfer, FEBS Lett., 237, 57–60.
Plato, M., Lendzian, F., Lubitz, W., and Mobius, K. (1992) Molecular orbital study of electronic asymmetry in primary donors of bacterial reaction centers, in The Photosynthetic Bacterial Reaction Center II: Structure, Spectroscopy, and Dynamics (Breton, J., and Vermeglio, A., eds.) Plenum, New York, pp. 109–118.
Hamm, P., and Zinth, W. (1995) Ultrafast initial reaction in bacterial photosynthesis revealed by femtosecond infrared spectroscopy, J. Phys. Chem., 99, 13537–13544.
Lyle, P. A., Kolaczkowski, S. V., and Small, G. J. (1993) Photochemical hole-burned spectra of protonated and deuterated reaction centers of Rhodobacter sphaeroides, J. Phys. Chem., 97, 6924–6933.
Khatypov, R. A., Khmelnitskiy, A. Yu., Khristin, A. M., Fufina, T. Yu., Vasilieva, L. G., and Shuvalov, V. A. (2012) Primary charge separation within P870* in wild type and heterodimer mutants in femtosecond time domain, Biochim. Biophys. Acta, 1817, 1392–1398.
Warshel, A., and Parson, W. W. (1987) Spectroscopic properties of photosynthetic reaction centers. 1. Theory, J. Am. Chem. Soc., 109, 6143–6152.
Parson, W. W., and Warshel, A. (2004) Dependence of photosynthetic electron-transfer kinetics on temperature and energy in a density-matrix model, J. Phys. Chem., 108, 10474–10483.
Renger, T. (2004) Theory of optical spectra involving charge transfer states: dynamic localization predicts a temperature dependent optical band shift, Phys. Rev. Lett., 93, 1–4.
Yakovlev, A. G., Vasilieva, L. G., Shkuropatov, A. Ya., Bolgarina, T. I., Shkuropatova, V. A., and Shuvalov, V. A. (2003) Mechanism of charge separation and stabilization of separated charges in reaction centers of Chloroflexus aurantiacus and of YM210W(L) mutants of Rhodobacter sphaeroides excited by 20 fs pulses at 90 K, J. Phys. Chem., 107, 8330–8338.
Struve, W. S. (1995) Vibrational equilibration in absorption difference spectra of chlorophyll a, Biophys. J., 69, 2739–2744.
Yakovlev, A. G., and Shuvalov, V. A. (2015) Spectral exhibition of electron-vibrational relaxation in P* state of Rhodobacter sphaeroides reaction centers, Photosynth. Res., 125, 9–22.
Parshakov, A. N. (2010) Physics of Oscillations [in Russian], Perm State University, Perm, p. 302.
Milanovsky, G. E., Shuvalov, V. A., Semenov, A. Yu., and Cherepanov, D. A. (2015) Elastic vibrations in the photosynthetic bacterial reaction center coupled to the primary charge separation: implications from molecular dynamics simulations and stochastic Langevin approach, J. Phys. Chem., 119, 13656–13667.
Eisenmayer, T. J., De Groot, H. J. M., Van de Wetering, E., Neugebauer, J., and Buda, F. (2012) Mechanism and reaction coordinate of directional charge separation in bacterial reaction centers, J. Phys. Chem. Lett., 3, 694–697.
Eisenmayer, T. J., Lasave, J. A., Monti, A., De Groot, H. J. M., and Buda, F. (2013) Proton displacements coupled to primary electron transfer in the Rhodobacter sphaeroides reaction center, J. Phys. Chem., 117, 11162–11168.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A. G. Yakovlev, V. A. Shuvalov, 2017, published in Biokhimiya, 2017, Vol. 82, No. 8, pp. 1176-1187.
Originally published in Biochemistry (Moscow) On-Line Papers in Press, as Manuscript BM17-158, July 17, 2017.
Rights and permissions
About this article
Cite this article
Yakovlev, A.G., Shuvalov, V.A. Femtosecond relaxation processes in Rhodobacter sphaeroides reaction centers. Biochemistry Moscow 82, 906–915 (2017). https://doi.org/10.1134/S0006297917080053
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297917080053