Abstract
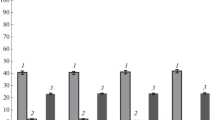
The biosynthesis of succinic acid from glucose by the previously engineered E. coli strain SUC1.0 (pMW119-kgd) (MG1655 ∆ackA-pta, ∆poxB, ∆ldhA, ∆adhE, ∆ptsG, PLglk, PtacgalP, ∆aceBAK, ∆glcB, ∆sdhAB, pMW119-kgd) was optimized. The yield of the target substance was increased, upon the activation in the strain of the tricarboxylic acid cycle variant mediated by the action of heterologous 2-ketoglutarate decarboxylase, due to the intensification of the anaplerotic formation of oxaloacetic acid. Inactivation of the nonspecific thioesterase YciA in the strain did not considerably change the biosynthetic characteristics of the producer. The enhancement of the expression of native phosphoenolpyruvate carboxylase led to an increase in the yield of the target compound by the recombinant synthesizing succinic acid via the reactions of the native tricarboxylic acid cycle from 25 to 42%, and from 67 to 75% upon the induced expression of Mycobacterium tuberculosis 2-ketoglutarate decarboxylase. The expression of the Bacillus subtilis pyruvate carboxylase gene in the strain resulted in an increase in the yield of succinic acid up to 84%. While functioning in whole-cell biocatalyst mode, the engineered strain SUC1.0 PL-pycA (pMW119-kgd) demonstrated a substrate-to-target product conversion ratio reaching 93%, approaching the corresponding theoretical maximum.

Similar content being viewed by others
REFERENCES
Mazzoli, R., Fermentation, 2021, vol. 7, no. 4, p. 248. https://doi.org/10.3390/fermentation7040248
Escanciano, I.A., Wojtusik, M., Esteban, J., Ladero, M., and Santos, V.E., Fermentation, 2022, vol. 8, no. 8, p. 368. https://doi.org/10.3390/fermentation8080368
Guettler, M.V., Rumler, D., and Jain, M.K., Int. J. Syst. Bacteriol., 1999, vol. 49, pp. 207–216.
Nghiem, N.P., Davison, B.H., Suttle, B.E., and Richardson, G.R., Appl. Biochem. Biotechnol., 1997, vol. 63–65, pp. 565–576.
Lee, P.C., Lee, S.Y., Hong, S.H., and Chang, H.N., Appl. Microbiol. Biotechnol., 2002, vol. 58, no. 5, pp. 663–668.
Liu, X., Zhao, G., Sun, S., Fan, C., Feng, X.M., and Xiong, P., Front. Bioeng. Biotechnol., 2022, vol. 10, p. 3389. https://doi.org/10.3389/fbioe.2022.843887
Skorokhodova, A.Yu., Gulevich, A.Yu., Morzhakova, A.A., Shakulov, R.S., and Debabov, V.G., Biotekhnologiya, 2012, no. 2, pp. 8–20.
Skorokhodova, A.Y., Morzhakova, A.A., Gulevich, A.Y., and Debabov, V.G., J. Biotechnol., 2015, vol. 214, pp. 33–42.
Lin, H., Bennett, G.N., and San, K.Y., Metab. Eng., 2005, vol. 7, no. 2, pp. 116–127.
Park, S.J., Chao, G., and Gunsalus, R.P., J. Bacteriol., 1997, vol. 179, no. 13, pp. 4138–4142.
Skorokhodova, A.Yu., Stasenko, A.A., Gulevich, A.Yu., and Debabov, V.G., App. Biochem. Microbiol., 2018, vol. 54, no. 3, pp. 245–251. https://doi.org/10.1134/S0003683818030134
Skorokhodova, A.Y., Gulevich, A.Y., and Debabov, V.G., Biotechnol. Rep., 2022, vol. 33, p. e00703. https://doi.org/10.1016/j.btre.2022.e00703
Skorokhodova, A.Y., Stasenko, A.A., Krasilnikova, N.V., Gulevich, A.Y., and Debabov, V.G., Fermentation, 2022, vol. 8, no. 12, p. 738. https://doi.org/10.3390/fermentation8120738
Tian, J., Bryk, R., Itoh, M., Suematsu, M., and Nathan, C., Proc. Natl. Acad. Sci. U. S. A., 2005, vol. 102, no. 30, pp. 10670–10675.
Zhang, S. and Bryant, D.A., Science, 2011, vol. 334, no. 6062, pp. 1551–1553.
Sambrook, J., Fritsch, E., and Maniatis, T., Molecular Cloning: A Laboratory Manual, New York: Cold Spring Harbor Lab. Press, 1989, 2nd ed.
Datsenko, K.A. and Wanner, B.L., Proc. Natl. Acad. Sci. U. S. A., 2000, vol. 97, no. 12, pp. 6640–6645.
Katashkina, Zh.I., Skorokhodova, A.Yu., Zimenkov, D.V., Gulevich, A.Yu., Minaeva, N.I., Doroshenko, V.G., Biryukova, I.V., and Mashko, S.V., Mol. Biol. (Moscow), 2005, vol. 39, no. 5, pp. 719–726.
Gulevich, A.Yu., Skorokhodova, A.Yu., and Debabov, V.G., App. Biochem. Microbiol., 2021, vol. 57, no. 2, pp. 161–169.
Gulevich, A.Yu., Skorokhodova, A.Yu., Ermishev, V.Yu., Krylov, A.A., Minaeva, N.I., Polonskaya, Z.M., Zimenkov, D.V., Biryukova, I.V., and Mashko, S.V., Mol. Biol. (Moscow), 2009, vol. 43, no. 3, pp. 505–514.
Gulevich, A.Yu., Skonechnyi, M.S., Sukhozhenko, A.V., Skorokhodova, A.Yu., and Debabov, V.G., Biotekhnologiya, 2015, no. 2, pp. 46–54.
Clomburg, J.M., Vick, J.E., Blankschien, M.D., Rodriguez-Moya, M., and Gonzalez, R., ACS Synth. Biol., 2012, vol. 1, pp. 541–554.
Jitrapakdee, S., St. Maurice, M., Rayment, I., Cleland, W.W., Wallace, J.C., and Attwood, P.V., Biochem. J., 2008, vol. 413, no. 3, pp. 369–387.
Skorokhodova, A.Yu., Gulevich, A.Yu., and Debabov, V.G., Biotekhnologiya, 2018, vol. 34, no. 2, pp. 18–25.
Chang, D.E., Shin, S., Rhee, J.S., and Pan, J.G., J. Bacteriol., 1999, vol. 181, no. 21, pp. 6656–6663.
Burgard, A., Burk, M.J., Osterhout, R., Van Dien, S., and Yim, H., Curr. Opin. Biotechnol., 2016, vol. 42, pp. 118–125.
Seol, W. and Shatkin, A.J., J. Biol. Chem., 1992, vol. 267, no. 9, pp. 6409–6413.
Funding
This work was supported by ongoing institutional funding. No additional grants to carry out or direct this particular research were obtained.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors of this work declare that they have no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This work does not contain any studies involving human and animal subjects.
Additional information
Translated by V. Mittova
Publisher’s Note.
Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Skorokhodova, A.Y., Gulevich, A.Y. & Debabov, V.G. Optimization of Aerobic Synthesis of Succinic Acid from Glucose by Recombinant Escherichia coli Strains through the Tricarboxylic Acid Cycle Variant Mediated by the Action of 2-Ketoglutarate Decarboxylase. Appl Biochem Microbiol 59, 786–792 (2023). https://doi.org/10.1134/S0003683823060169
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683823060169