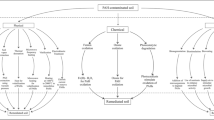
Abstract
The degradation of stable organophosphorus pollutants has been studied in six soil bacterial isolates and three strains of bacteria adapted to utilize glyphosate herbicide (GP) under laboratory conditions. Significant differences in the uptake of organophosphonates were found in taxonomically close strains possessing similar enzymatic pathways of catabolism of these compounds, which suggests the existence of unknown mechanisms for the regulation of the activity of these enzymes. The effect of adaptation to GP utilization as the sole phosphorus source on the consumption rates of several other structurally different phosphonates was observed in the studied bacteria. New, highly efficient degrading strains that resulted in a GP decomposition of up to 56% after soil application were isolated. Unsolved problems of microbial GP metabolism and trends in further research on the creation of effective preparations for the bioremediation of soils contaminated with organophosphonates are discussed.
Similar content being viewed by others
REFERENCES
White, A.K. and Metcalf, W.W., Microbial metabolism of reduced phosphorus compounds, Annu. Rev. Microbiol., 2007, vol. 61, pp. 379–400. https://doi.org/10.1146/annurev.micro.61.080706.093357
McGrath, J.W., Chin, J.P., and Quinn, J.P., Organophosphonates revealed: new insights into the microbial metabolism of ancient molecules, Nat. Rev. Microbiol., 2013, vol. 11, no. 6, pp. 412–419.
Ermakova, I.T., Shushkova, T.V., and Leont’evskii, A.A., Microbial degradation of organophosphonates by soil bacteria, Microbiology (Moscow), 2008, vol. 77, pp. 615–620.
Horsman, G.P. and Zechel, D.L., Phosphonate biochemistry, Chem. Rev., 2017, vol. 117, no. 8, pp. 5704–5783. https://doi.org/10.1021/acs.chemrev.6b00536
Benbrook, C.M., Trends in glyphosate herbicide use in the United States and globally, Environ. Sci. Eur., 2016, vol. 28, no. 3, pp. 1–15. https://doi.org/10.1186/s12302-016-0070-0
Kniss, A.R., Long-term trends in the intensity and relative toxicity of herbicide use, Nat. Commun., 2017, vol. 8, p. 14865. https://doi.org/10.1038/ncomms14865
Mesnag,e, R., Defarge, N., Spiroux de Vendomois, J., and Seralini, G.E., Potential toxic effects of glyphosate and its commercial formulations below regulatory limits, Food Chem. Toxicol., 2015, vol. 84, pp. 133–153. https://doi.org/10.1016/j.fct.2015.08.012
Bai, S.H. and Ogbourne, S.M., Glyphosate: environmental contamination, toxicity and potential risks to human health via food contamination, Environ. Sci. Pollut. Res. Int., 2016, vol. 23, no. 19, pp. 18988–19001. https://doi.org/10.1007/s11356-016-7425-3
Zhan, H., Feng, Y., Fan, X., and Chen, S., Recent advances in glyphosate biodegradation, Appl. Microbiol. Biotechnol., 2018, vol. 102, no. 12, pp. 5033–5043. https://doi.org/10.1007/s00253-018-9035-0
Sviridov, A.V., Epiktetov, D.O., Shushkova, T.V., et al., Fosfororganicheskie neirotoksiny (Organophosphate Neurotoxins), Moscow: RIOR, 2020.
Fan, J., Yang, G., Zhao, H., et al., Isolation, identification and characterization of a glyphosate-degrading bacterium, Bacillus cereus CB4, from soil, J. Gen. Appl. Microbiol., 2012, vol. 58, no. 4, pp. 263–271. https://doi.org/10.2323/jgam.58.263
Yu, X.M., Yu, T., Yin, G.H., et al., Glyphosate biodegradation and potential soil bioremediation by Bacillus subtilis strain Bs-15, Genet. Mol. Res., 2015, vol. 14, no. 4, pp. 14717–14730. https://doi.org/10.4238/2015
Huntscha, S., Stravs, M.A., Buhlmann, A., et al., Seasonal dynamics of glyphosate and AMPA in lake Greifensee: rapid microbial degradation in the epilimnion during summer, Environ. Sci. Technol., 2018, vol. 52, no. 8, pp. 4641–4649. https://doi.org/10.1021/acs.est.8b00314
Acosta-Cortés, A.G., Martinez-Ledezma, C., López-Chuken, U.J., et al., Polyphosphate recovery by a native Bacillus cereus strain as a direct effect of glyphosate uptake, ISME J., 2019, vol. 13, no. 6, pp. 1497–1505. https://doi.org/10.1038/s41396-019-0366-3
Ermakova, I.T. Kiseleva, N.I., et al., Bioremediation of glyphosate-contaminated soils, Appl. Microbiol. Biotechnol., 2010, vol. 88, no. 2, pp. 585–594. https://doi.org/10.1007/s00253-010-2775-0
Hove-Jensen, B., Zechel, D.L., and Jochimsen, B., Utilization of glyphosate as phosphate source: biochemistry and genetics of bacterial carbon-phosphorus lyase, Microbiol. Mol. Biol. Rev., 2014, vol. 78, no. 1, pp. 176–197. https://doi.org/10.1128/MMBR.00040-13
Lyagin, I.V., Andrianova, M.S., and Efremenko, E.N., Extensive hydrolysis of phosphonates as unexpected behavior of the known His6-organophosphorus hydrolase, Appl. Microbiol. Biotechnol., 2016, vol. 100, no. 13, pp. 5829–5838. https://doi.org/10.1007/s00253-016-7407-x
Villareal-Chiu, J.F., Quinn, J.P., and McGrath, J.W., The genes and enzymes of phosphonate metabolism by bacteria, and their distribution in the marine environment, Front. Microbiol., 2012, vol. 3, no. 19, pp. 1–13. https://doi.org/10.3389/fmicb.2012.00019
Sosa, O.A., Repeta, D.J., DeLong, E.F., et al., Phosphate-limited ocean regions select for bacterial populations enriched in the carbon-phosphorus lyase pathway for phosphonate degradation, Environ. Microbiol., 2019, vol. 21, no. 7, pp. 2402–2414. https://doi.org/10.1111/1462-2920.14628
Sviridov, A.V., Shushkova, T.V., Ermakova, I.T., et al., Microbial degradation of glyphosate herbicides (review), Appl. Biochem. Microbiol., 2015, vol. 51, no. 2, pp. 188–195.
Kishore, G.M. and Barry, G.F., Glyphosate tolerant plants. US Patent no. US5463175A, 1995.
Pedotti, M., Rosini, E., Molla, G., et al., Glyphosate resistance by engineering the flavoenzyme glycine oxidase, J. Biol. Chem., 2009, vol. 284, no. 51, pp. 36415–36423.
Yao, P., Lin, Y., Wu, G., et al., Improvement of glycine oxidase by DNA shuffling, and site-saturation mutagenesis of F247 residue, Int. J. Biol. Macromol., 2015, vol. 79, pp. 965–970. https://doi.org/10.1016/j.ijbiomac.2015.05.030
Castle, L.A., Siehl, D.L., Gorton, R., et al., Discovery and directed evolution of a glyphosate tolerance gene, Science, 2004, vol. 304, no. 5674, pp. 1151–1154.
Shushkova, T.V., Vinokurova, N.G., Baskunov, B.P., et al., Glyphosate acetylation as a specific trait of Achromobacter sp. Kg 16 physiology, Appl. Microbiol. Biotechnol., 2016, vol. 100, no. 2, pp. 847–855. https://doi.org/10.1007/s00253-015-7084-1
Kertesz, M., Elgorriaga, A., and Amrhein, N., Evidence for two distinct phosphonate-degrading enzymes (C-P lyases) in Arthrobacter sp. GLP-1, Biodegradation, 1991, vol. 2, no. 1, pp. 53–59. https://doi.org/10.1007/bf00122425
Sviridov, A.V., Shushkova, T.V., Zelenkova, N.F., et al., Distribution of glyphosate and methylphosphonate catabolism systems in soil bacteria Ochrobactrum anthropi and Achromobacter sp., Appl. Microbiol. Biotechnol., 2012, vol. 93, no. 2, pp. 787–796.
Ermakova, I.T., Shushkova, T.V., Sviridov, A.V., et al., Organophosphonates utilization by soil strains of Ochrobactrum anthropi and Achromobacter sp., Arch. Microbiol., 2017, vol. 199, no. 5, pp. 665–675.
Sviridov, A.V., Zelenkova, N.F., Vinokurova, N.G., et al., New approaches to identification and activity estimation of glyphosate degradation enzymes, Biochemistry (Moscow), 2011, vol. 76, no. 6, pp. 720–725. https://doi.org/10.1134/S0006297911060149
Spilker, T., Vandamme, P., and LiPuma, J.J., A multilocus sequence typing scheme implies population structure and reveals several putative novel Achromobacter species, J. Clin. Microbiol., 2012, vol. 50, no. 9, pp. 3010–3015. https://doi.org/10.1128/JCM.00814-12
Spilker, T., Vandamme, P., and LiPuma, J.J., Identification and distribution of Achromobacter species in cystic fibrosis, J. Cyst. Fibros., 2013, vol. 12, no. 3, pp. 298–301. https://doi.org/10.1016/j.jcf.2012.10.002
Bolger, A.M., Lohse, M., and Usadel, B., Trimmomatic: a flexible trimmer for Illumina sequence data, Bioinformatics, 2014, vol. 30, no. 15, pp. 2214–2120. https://doi.org/10.1093/bioinformatics/btu170
Bankevich, A., Nurk, S., Antipov, D., et al., SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing, J. Comput. Biol., 2012, vol. 19, no. 5, pp. 455–477. https://doi.org/10.1089/cmb.2012.0021
Tatusova, T., DiCuccio, M., Badretdin, A., et al., NCBI Prokaryotic genome annotation pipeline, Nucleic Acids Res., 2016, vol. 44, no. 14, pp. 6614–6624. https://doi.org/10.1093/nar/gkw569
Shushkova, T.V., Vasilieva, G.K., Ermakova, I.T., and Leontievsky, A.A., Sorption and microbial degradation of glyphosphate in soil suspensions, Appl. Biochem. Microbiol., 2009, vol. 45, no. 6, pp. 599–603. https://doi.org/10.1134/S0003683809060040
Gimsing, A.L., Borggaard, O.K., Jacobsen, O.S., et al., Chemical and microbiological soil characteristics controlling glyphosate mineralization in Danish surface soils, Appl. Soil Ecol., 2004, vol. 27, no. 3, pp. 233–242. https://doi.org/10.1016/j.apsoil.2004.05.007
Hadi, F., Mousavi, A., Noghabi, K.A., et al., New bacterial strain of the genus Ochrobactrum with glyphosate-degrading activity, J. Environ. Sci. Health B, 2013, vol. 48, no. 3, pp. 208–213. https://doi.org/10.1080/03601234.2013.730319
Firdous, S., Iqbal, S., Anwar, S., and Jabeen, H Identification and analysis of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene from glyphosate-resistant Ochrobactrum intermedium Sq20, Pest Manage. Sci., 2018, vol. 74, no. 5, pp. 1184–1196. https://doi.org/10.1002/ps.4624
Bazot, S. and Lebeau, T., Simultaneous mineralization of glyphosate and diuron by a consortium of three bacteria as free-and/or immobilized-cells formulations, Appl. Microbiol. Biotechnol., 2008, vol. 77, no. 6, pp. 1351–1358. https://doi.org/10.1007/s00253-007-1259-3
Vandamme, P., Moore, E.R., Cnockaert, M., et al., Classification of Achromobacter genogroups 2, 5, 7 and 14 as Achromobacter insuavis sp. nov., Achromobacter aegrifaciens sp. nov., Achromobacter anxifer sp. nov. and Achromobacter dolens sp. nov., respectively, Syst. Appl. Microbiol., 2013, vol. 36, no. 7, pp. 474–482. https://doi.org/10.1016/j.syapm.2013.06.005
Zhang, G., Mazurkie, A.S., Dunaway-Mariano, D., and Allen, K.N., Kinetic evidence for a substrate-induced fit in phosphonoacetaldehyde hydrolase, Biochemistry, 2002, vol. 41, no. 45, pp. 13370–13377. https://doi.org/10.1021/bi026388n
Quinn, J.P., Kulakova, A.N., Cooley, N.A., and McGra-th, J.W., New ways to break an old bond: the bacterial carbonphosphorus hydrolases and their role in biogeochemical phosphorus cycling, Environ. Microbiol., 2007, vol. 9, no. 10, pp. 2392–2400. https://doi.org/10.1111/j.1462-2920.2007.01397.x
Funding
The phosphonatase studies and whole genome sequencing were supported by the Russian Science Foundation, grant no. 18-074-00021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
This article does not contain any studies involving animals performed by any of the authors.
This article does not contain any studies involving human participants performed by any of the authors.
Additional information
Translated by I. Gordon
Abbreviations: 2-AEP—2-aminoethylphosphonate; AMPA—aminomethylphosphonic acid; CFU—colony-forming unit(s); dNTP—deoxyribonucleoside triphosphate; FMN—fosfomycin; GAT—glyphosate acetyl transferase; GOX—glyphosate oxidoreductase; GP—glyphosate; HPLC—high performance liquid chromatography; MLST—multilocus sequence typing; MPA—methylphosphonic acid; NADH—nicotinamide adenine dinucleotide (reduced); OP—organophosphonates; PA—phosphonoacetate; PAH—phosphonoacetate hydrolase; PCR—polymerase chain reaction; Pi—orthophosphate; PNT—phosphonatase (phosphonoacetaldehyde hydrolase).
Rights and permissions
About this article
Cite this article
Sviridov, A.V., Shushkova, T.V., Epiktetov, D.O. et al. Biodegradation of Organophosphorus Pollutants by Soil Bacteria: Biochemical Aspects and Unsolved Problems. Appl Biochem Microbiol 57, 836–844 (2021). https://doi.org/10.1134/S0003683821070085
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683821070085