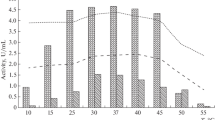
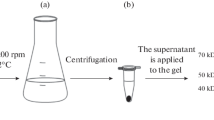
Abstract
The peptidase activity of the alkaliphilic, aerobic, proteolytic bacteria Alkalicaulis satelles G-192T and Aliidiomarina sp. P-156 isolated from the system of hypersaline, alkaline Tanatar lakes (Altai Territory) was studied. Strains G-192 and P-156 710 were shown to hydrolyze para-nitroanilide substrates and exhibit the highest activity hydrolyzing of the aminopeptidase LpNa substrate. Analysis of partially purified peptidase preparations showed that the enzymes were most active and stablest in an alkaline pH range of 8.4–11. The peptidases of strains G-192 and P-156 were highly stable in NaCl up to 220 and 70 g/L respectively. The results of inhibitor analysis and the substrate specificity of the studied extracellular enzymes indicated their classification as metallopeptidases of the aminopeptidase type. The studied peptidases showed significant resistance to the surfactants Triton X-100 and SDS and the oxidizing agent H2O2. The isolated bacteria that produce peptidases can be used as a source of proteolytic enzymes in the development of new detergents.




Similar content being viewed by others
REFERENCES
Luo, L., Meng, H., and Gu, J-D., J. Environ. Manage., 2017, vol. 197, pp. 539–549.
Ibrahim, A.S.S., Al-Salamah, A.A., El-Badawi, Y.B., El-Tayeb, M.A., and Antranikian, G., Extremophiles, 2015, vol. 19, no. 5, pp. 961–971.
Garg, S.K. and Singh, K.S., Enz. Eng., 2015, vol. 4, no. 2. Art. 129. https://doi.org/10.4172/2329-6674.1000129
Sharma, M., Gat, Y., Arya, S., Kumar, V., Panghal, A., and Kumar, A.A., Ind. Biotechnol., 2019, vol. 15, no. 2, pp. 69–78.
Zhang, H., Li, H., Lang, D.A., Xu, H., and Zhu, H., J. Chem. Technol. Biotechnol., 2018, vol. 93, pp. 3627–3637.
Zhou, C., Qin, H., Chen, X., Zhangy, Xue, Y., and Ma, Y., Sci Rep., 2018, vol. 8. Art. 16467. https://doi.org/10.1038/s41598-018-34416-5
Uma, G., Babu, M.M., Prakash, V.S.G., Nisha, S.J., and Citarasu, T., J. Microbiol. Biotechnol., 2020, vol. 36, no. 5. Art. 66. https://doi.org/10.1007/s11274-020-02841-2
Danilova, I. and Sharipova, M., Front. Microbiol., 2020, vol. 11, p. 1782. https://doi.org/10.3389/fmicb.2020.01782
Gessesse, A. and Gashe, B.A., Biotechnol. Lett., 1997, vol. 19, pp. 479–481.
Nilegaonkar, S., Kanekar, P., Sarnaik, S., and Kelkar, A.S., J. Microbiol. Biotechnol., 2002, vol. 18, pp. 785–789.
Karan, R., Singh, S.P., Kapoor, S., and Khare, S.K., N. Biotechnol., 2011, vol. 28, no. 2, pp. 136–145.
Ibrahim, A.S., Al-Salamah, A.A., El-Badawi, Y.B., El-Tayeb, MohamedA., and Ibrahim, S.S., Biosci. J., 2016, vol. 32, no. 6, pp. 1604–1618.
Rathod, M.G. and Pathak, A.P., Data Brief, 2016, vol. 8, pp. 863–866.
Abdel-Hamed, A.R., Abo-Elmatty, D.M., Wiegel, J., and Mesbah, N.M., Extremophiles, 2016, vol. 20, pp. 885–894.
Kevbrin, V., Boltyanskaya, Y., Koziaeva, V., Uzun, M., and Grouzdev, D., Int. J. Syst. Evol. Microbiol., 2021, vol. 71, no. 1. https://doi.org/10.1099/ijsem.0.004614
Erlanger, B.F., Kokowsky, N., and Cohen, W., Arch. Biochem. Biophys., 1961, vol. 95, no. 2, pp. 271–278.
Patel, A.R., Mokashe, N.U., Chaudhari, D.S., Jadhav, A.G., and Patil, U.K., Biocatal. Agric. Biotechnol., 2019, vol. 19, pp. 101–122.
Ibrahim, A.S.S., Elbadawi, Y.B., Tayeb, M.A.E., Maary, K.S.A., Maany, D.A.F., Ibrahim, S.S.S., and Elagib, A.A., 3 Biotech., 2019, vol. 9, no. 11, p. 391.
Abu-Khudir, R., Salem, M.M., Allam, N.G., and Ali, E.M.M., Appl. Biochem. Biotechnol., 2019, vol. 189, no. 1, pp. 87–102.
Yildirim, V., Baltaci, M.O., Ozgencli, I., Sisecioglu, M., Adiguzel, A., and Adiguzel, G., J. Enzyme Inhib. Med. Chem., 2017, vol. 32, no. 1, pp. 468–477.
Mokashe, N., Chaudhari, B., and Patil, U., J. Surfactants Deterg., 2017, vol. 20, no. 6, pp. 1377–1393.
Mothe, T. and Sultanpuram, V., 3 Biotech., 2016, vol. 6, no. 53. https://doi.org/10.1007/s13205-016-0377-y
Gupta, R., Beg, Q., and Lorenz, P., Appl. Microbiol. Biotechnol., 2002, vol. 59, pp. 15–32.
Jaouadi, N.Z., Jaouadi, B., Aghajari, N., and Bejar, S., Process Biochem., 2012, vol. 105, pp. 142–151.
Hellmuth, H. and Dreja, M., Tenside Surfactants Deterg., 2016, vol. 53, pp. 502–508.
Holmberg, K., Colloids Surf. B Biointerfaces, 2018, vol. 168, pp. 169–177.
Shaikh, I.K., Dixit, P.P., and Shaikh, T.M., J. Genet. Eng. Biotechnol., 2018, vol. 16, no. 2, pp. 273–279.
Funding
This work was carried out with the financial support of the Russian Foundation for Basic Research, project no. 18-04-00236, partly within the framework of the state assignment AAAA-A19-119020590109-3 for the Federal Research Center of Biotechnology of the Russian Academy of Sciences and partly within the framework of the state assignment 0271-2021-0003 (FWSM-2021-0003) for a Federal State Budgetary Institution of Science, the Institute of General and Experimental Biology of the Siberian Branch of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lavrentyeva, E.V., Erdyneeva, E.B., Dunaevskii, Y.E. et al. Extracellular, Highly Stable, Alkaline Peptidases of the Alkalophilic Bacteria Alkalicaulis satelles G-192t and Aliidiomarina sp. P-156 and Their Possible Use in the Composition of Detergents. Appl Biochem Microbiol 57, 725–731 (2021). https://doi.org/10.1134/S0003683821060089
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683821060089