Abstract
Human gene therapy (HGT) aims to cure disease by inserting or editing the DNA of patients with genetic conditions. Since foundational genetic techniques came into use in the 1970s, the field has developed to the point that now three therapies have market approval, and over 1800 clinical trials have been initiated. In this article I present a brief history of HGT, showing how the ethical and practical viability of the field was achieved by key scientific and regulatory actors. These parties carefully articulated gene therapy’s scope, limiting it to therapeutic interventions on somatic cells, and cultivated alliances and divisions that bolstered the field’s legitimacy. At times these measures faltered, and then practitioners and sometimes patients would invoke an ethical imperative, posing gene therapy as the best solution to life and death problems. I suggest that we consider how boundary-work stretches out from science to enlist diverse publics, social formations and the natural world in the pursuit of legitimacy.
Similar content being viewed by others
Notes
I use ‘gene transfer’ and ‘gene therapy’ interchangeably throughout this article.
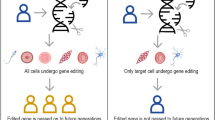
Another case from which to explore these questions is that of CRISPR/Cas-9 and associated gene editing techniques (TALENs, Zinc Finger Nucleases). Gene editing can theoretically make ultra-precise (single base) changes to the genome of almost any organism, suggesting the potential for safer and more accurate gene therapy. In April of this year a publication reported that CRISPR/Cas-9 was used in non-viable embryos with little success (Liang et al, 2015), sparking an uproar that rippled out of the gene therapy community and into the biosciences more broadly. This primary concern was the application of CRISPR/Cas-9 in embryos, where any changes induced would be passed on to subsequent generations (though in this case the embryos were terminated soon after the experiment). The case instigated an ultimately unsuccessful call for a moratorium on gene editing, investigations from the Nuffield Council on Bioethics in the United Kingdom and the National Academies of Sciences and Medicine in the United States, and culminated in an international summit held in December 2015, where technical, ethical and social issues were discussed. The debate has frequently conflated gene editing (the host of technologies) with germ-line interventions (applying those technologies to germ cells), and points to both the vulnerability of the germ line as a boundary, and the weight of concern and meaning the germ line bears.
Notable exceptions are Paul Martin (1999), who shows among other things how gene therapy was coproduced with newly geneticised disease concepts, and Wailoo and Pemberton (2006) who explore genetic medicine more generally in relation to Tay Sachs, Sickle Cell Disease and CF.
Martin (1999) suggests that public concerns over genetic modification arose from a broader, growing discontent with science and its promises, as well increasing opposition to contemporary political events such as the Vietnam War.
Their concern was that this technology might have consequences for human health and the ecosystem, perhaps by creating new pathogens (Berg and Singer, 1995).
Beta thalassaemia was an early HGT target because it was well characterised, with a known genetic cause (the beta-globin gene) and effect (an inadequate amount of beta-globin chains are produced, causing anaemia) (Anderson and Fletcher, 1980).
The President’s Commission produced concrete results and included the formation of an RAC subcommittee for HGT, which issued recommendations through a ‘Points to Consider’ document, and provided prospective researchers advice (O’Reilly et al, 2013).
The disease is characterised by one dysfunctional gene for the enzyme adenosine deaminase. Pre-clinical research in human cells, mice and non-human primates suggested that the team’s method held promise (Culver et al, 1991).
The primary concern of Churchill et al (1998) is how this blurring compromises informed consent in gene therapy, and is well worth reading.
The EWGT federated scientists from 18 European countries, and survives as the European Society of Gene and Cell Therapy (Cohen-Haguenauer, 1992).
Susan Lindee points out that gene therapy was a commonly invoked end goal of genome mapping. In reality, the primary technological intervention the HGP has facilitated is abortion of afflicted embryos.
The RACs’ remit was reduced on the suggestion of two advisory groups. Some felt the layer of oversight they provided impeded authorisations, others that it was simply unnecessary, since human trials had been underway for 5 years without serious safety issues (Marshall, 1996).
For a review of vector types and their properties, see O’Reilly et al (2013).
Often the immune response recognises the inserted gene as foreign and inhibits it; furthermore, many genes need their expression to be regulated, itself a complicated undertaking.
A London-based X-SCID trial later reported similar results in their 10 patients, whose T-cell counts improved, though their humoral immunity remained below average (Gaspar et al, 2011).
For an overview of European gene therapy regulation, see Klug et al (2012).
mtDNA is DNA found outside the cell nucleus, and is passed down the maternal lineage (that is, children only inherit their mothers’ mtDNA).
See HFEA (2014) for detail about how these two techniques work.
Whether or not mtDNA transfer counts as gene therapy depends on who is asked. I have chosen to use it in this discussion because what counts as gene therapy more broadly is up for debate, and this particular case continues earlier conversations originating from gene therapy, most notably those concerned with the status of the germ line.
References
AdSAT Working Group (2002) Assessment of adenoviral vector safety and toxicity: Report of the national institutes of health Recombinant DNA Advisory Committee (NIH Report). Human Gene Therapy 13(1): 3–13.
Allan, D.S. and Dubé, I.D. (1996) Symposium highlights: Future prospects for gene therapy. Transfusion Science 17(1): 203–205.
Amati, M.P. et al (2003) EMEA and gene therapy medicinal products development in the European Union. Journal of Biomedicine and Biotechnology 2003(1): 3–8.
Anderson, W.F. and Fletcher, J.C. (1980) Gene therapy in human beings: When is it ethical to begin? New England Journal of Medicine 303(22): 1293–1297.
Anderson, W.F. (1982) Human Genetic Engineering: Hearings before the Subcommittee on Investigations and Oversight of the Committee on Science and Technology. (Testimonial) U.S. House of Representatives, 97th Congress, 2nd Session, no. 170, Washington DC.
Anonymous (1988) Gene therapy in man: Recommendations of the European medical research councils. The Lancet 331(8597): 1271–1272.
Anonymous (2002) The trials of gene therapy. Nature 420(6912): 107.
Barber, S. and Border, P. (2015) Mitochondrial donation. (Standard Note SN/SC/6833) House of Commons Library.
Batshaw, M.L., Wilson, J.M., Raper, S., Yudkoff, M. and Robinson, M.B. (1999) Recombinant adenovirus gene transfer in adults with partial Ornithine Transcarbamylase deficiency (OTCD). Human Gene Therapy 10(14): 2419–2437.
Berg, P. et al (1974) Potential biohazards of recombinant DNA molecules. Science 185(4148): 303.
Berg, P. and Singer, M.F. (1995) The recombinant DNA controversy: Twenty years later. Proceedings of the National Academy of Sciences 92(20): 9011–9013.
Blaese, M.R. et al (1999) T-lymphocyte-directed gene therapy for ADA-SCID: Initial trial results after four years. Science 270(5235): 475–480.
Bordignon, C. et al (1993) Clinical protocol: Transfer of the ADA gene into bone marrow cells and peripheral blood lymphocytes for the treatment of patients affected by ADA-deficient SCID. Human Gene Therapy 4(4): 513–520.
Bordignon, C. et al (1995) Gene therapy in peripheral blood lymphocytes and bone marrow for ADA immunodeficient patients. Science 270(5235): 470–475.
Bowker, G.C. and Star, S.L. (1999) Sorting Things Out: Classification and its Consequences. Cambridge, MA: MIT Press.
Bryant, L.M. et al (2013) Lessons learned from the clinical development and market authorization of Glybera. Human Gene Therapy Clinical Development 24(2): 55–64.
Büning, H. (2013) Gene therapy enters the pharma market: The short story of a long journey. EMBO Molecular Medicine 5(1): 1–3.
Butler, D. (1994) Call for risk/benefit study of gene therapy. Nature 372(6508): 716.
Campbell, P., Maranto, G., Cantor, C.R., Glantz, L.H. and Miller, F.H. (1998) Gene therapy: Legal, financial and ethical issues. Boston University Journal of Science and Technology Law 4(3): 110.
Cavazzana-Calvo, M. et al (2000) Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288(5466): 669.
Cavazzana-Calvo, M.A., Thrasher, A. and Mavilio, F. (2004) The future of gene therapy: Balancing the risks and benefits of clinical trials. Nature 427(6977): 779–781.
Check, E. (2003) Harmful potential of viral vectors fuels doubt over gene therapy. Nature 423(6940): 573–574.
Churchill, L.R., Collins, M.L., King, N.M.P., Pemberton, S.G. and Wailoo, K.A. (1998) Genetic research as ‘therapy’: Implications of ‘gene therapy’ for informed consent. Journal of Law, Medicine and Ethics 26(1): 38–47.
Clothier Committee (1992) Report of the committee on the ethics of gene therapy. Human Gene Therapy 3(5): 519–523.
Cohen-Haguenauer, O. (1992) Gene therapy in Europe. Transfusion Science 17(1): 185–190.
Comfort, N. (2013) The Science of Human Perfection: How Genes became the Heart of American Medicine. New Haven, CT, London: Yale University Press.
Committee on Government Reforms (2000) Human Subject Research Protections. Hearing before the Subcommittee on Criminal Justice, Drug Policy and Human Resources of the Committee on Government Reforms. Washington DC: US Government Printing Office.
Couzin, J. and Kaiser, J. (2005) As Gelsinger case ends, gene therapy suffers another blow. Science 307(5712): 1028.
Culver, K.W. et al (1991) Correction of ADA deficiency in human T-lymphoctyes using retroviral-mediated gene transfer. Transplantation Proceedings. 23(1): 170–171.
Curnutte, M. and Testa, G. (2010) Consuming genomes: Scientific and social innovation in direct-to-consumer genetic testing. New Genetics and Society 31(2): 159–181.
Deakin, C.T., Alexander, I.E. and Kerridge, I. (2009) Accepting risk in clinical research: Is the gene therapy field becoming too risk averse? Molecular Therapy 17(11): 1842–1848.
Devlin, H. (2015) Britain’s House of Lords approves conception of three-parent babies. The Guardian, 24 February, http://www.theguardian.com/politics/2015/feb/24/uk-house-of-lords-approves-conception-of-three-person-babies.
Dworkin, R.B. (1990) Science, society, and the expert town meeting: Some comments on Asilomar. Southern California Law Review 51(6): 1471–1482.
Edelstein, M.L., Abedi, M.R. and Wixon, J. (2007) Gene therapy clinical trials worldwide to 2007 – An update. The Journal of Gene Medicine 9(10): 833–842.
Ehrich, K., Williams, C., Scott, R., Sandall, J. and Farsides, B. (2006) Social welfare, genetic welfare? Boundary work in the IVF-PGD clinic. Social Science and Medicine 63(5): 1213–1224.
EMA (European Medicines Agency) (2012) Glybera, alipogene tiparvovec. EPAR summary for the public, pp. 1–3.
Emery, D.W. (2004) Gene therapy for genetic disease: On the horizon. Clinical and Applied Immunology Reviews 4(6): 411–422.
Epstein, S. (1995) Impure Science: AIDS, Activism and the Politics of Knowledge. Berkeley, CA: University of California Press.
European Parliament and the Council of the European Union (2001) Directive 2001/20/EC.
Finkler, K. (2000) Experiencing the New Genetics: Family and Kinship on the Medical Frontier. Philadelphia, PA: University of Pennsylvania Press.
Fischer, M.M.J. (2004) Emergent Forms of Life and the Anthropological Voice. Durham, NC: Duke University Press.
Fletcher, J.C. (1990) Evolution of ethical debate about human gene therapy. Human Gene Therapy 1(1): 55–68.
Fortun, M. (2008) Promising Genomics: Iceland and deCODE Genetics in a World of Speculation. Berkeley, CA: University of California Press.
Franklin, S. and Roberts, C. (2006) Born and Made: An Ethnography of Pre-Implantation Genetic Diagnosis. Princeton, NJ; Oxford: Princeton University Press.
Frederikson, D.S. (1991) Asilomar and recombinant DNA: The end of the beginning. In: K.E. Hanna (ed.) Biomedical Politics. Washington DC: National Academies Press, pp. 258–298.
Friedman, T. (1992) A brief history of human gene therapy. Nature Genetics 2(2): 93–98.
Frist, B. (2002) Protecting Human Subjects in Research: Are Current Safeguards Accurate? Hearing before the Subcommittee on Public Health of the Committee of Health, Education, Labour and Pensions United States Senate. Washington DC: US Government Printing Office.
Frith, L., Jacoby, A. and Gabbay, M. (2011) Ethical boundary work in the infertility clinic. Sociology of Health and Illness 33(4): 570–585.
Gaspar, H.B. et al (2011) Long-term persistence of polyclonal T cell repertoire after gene therapy for X-linked severe combined immunodeficiency. Science Translational Medicine 3(97): 97–99.
Gibbon, S. (2002) Re-examining geneticization: Family trees in breast cancer genetics. Science as Culture 11(4): 429–457.
Gieryn, T. (1983) Boundary-work and the demarcation of science from non-science: Strains and interests in the professional ideologies of scientists. American Sociological Review 48(6): 781–795.
Gieryn, T. (1991) Cultural Boundaries of Science: Credibility on the Line. Chicago, IL: University of Chicago Press.
Ginn, S.L., Alexander, I.E., Edelstein, M.L., Abedi, M.R. and Wixon, J. (2013) Gene therapy clinical trials worldwide to 2012 – An update. Journal of Gene Medicine 15(2): 65–77.
Genetic Alliance UK (2014) Avoiding mitochondrial disease. Response to the Nuffield Council of Bioethics, pp. 1–3.
Gershon, D. (1990) Clinical trials next step. Nature 344(6261): 2.
Gore, A. (1982) Human Genetic Engineering: Hearings before the Subcommittee on Investigations and Oversight of the Committee on Science and Technology. Opening address, U.S. House of Representatives, 97th Congress, 2nd Session, no. 170, Washington DC.
Gore, A. (1984) Human Gene Therapy: A Background Paper. Washington DC: U.S. Congree, Office of Technology Assessment.
Grobhans, H. (2000) Gene therapy – When a simple concept meets a complex reality. Functional Integrative Genomics 1(2): 142–145.
Hacein-Bay-Abina, S. et al (2008) Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. Journal of Clinical Investigation 118(9): 3132–3142.
Hacein-Bay-Abina, S., von Kalle, C. and Schmidt, M. (2003a) A serious adverse event after successful gene therapy for X-Linked severe combined immunodeficiency. New England Journal of Medicine 348(3): 255–256.
Hacein-Bay-Abina, S. et al (2003b) LMO2-associated clonal T-cell proliferation in two patients after gene therapy for SCID-X1. Science 302(5644): 415–419.
Hedgecoe, A. (2001) Schitzophrenia and the narrative of enlightened geneticisation. Social Studies of Science 31(6): 875–911.
Horst, M. (2007) Public expectations of gene therapy: Scientific futures and their performative effects on scientific citizenship. Science, Technology and Human Values 32(2): 150–171.
House of Commons Science and Technology Committee (2014) Mitochondrial donation. Correspondence relating to evidence hearing, 22 October.
Human Fertilisation and Embryo Authority (2014) Mitochondrial donation: An introductory briefing note, http://www.hfea.gov.uk/6896.html.
Inhorn, M.C. and Birenbaum-Carmeli, D. (2008) Assisted reproductive technologies and culture change. Annual Review of Anthropology 37: 177–196.
Isasi, R.M., Nguyen, T.M. and Knoppers, B.M. (2006) National Regulatory Frameworks Regarding Human Genetic Modification Technologies. A report for the Genetics and Public Policy Centre, Montreal.
Kaiser, J. (2005) Panel urges limits on X-SCID trials. Science 307(5715): 1544–1545.
Kay, L.E. (2000) Who Wrote the Book of Life? A History of the Genetic Code. Stanford, CA: Stanford University Press.
Keller, E.F. (2000) The Century of the Gene. Cambridge, MA: Harvard University Press.
Kerr, A., Cunningham-Burley, S. and Amos, A. (1998) Eugenics and the new genetics in Britain: Examining contemporary professionals accounts. Science, Technology and Human Values 23(2): 175–198.
Kerr, A. and Shakespeare, T. (2002) Genetic Politics: From Genetics to Genome. Cheltenham, UK: New Clarion Press.
Kimmelman, J. (2009) Gene Transfer and the Ethics of First-in-Human Research: Lost in Translation. Cambridge, UK: Cambridge University Press.
Kim, S., Peng, Z. and Kaneda, Y. (2007) Current status of gene therapy in Asia. Molecular Therapy 16(2): 237–243.
Klug, B., Celis, P., Carr, M. and Reinhardt, J. (2012) Regulatory structures for gene therapy medicinal products in the European Union. Methods in Enzymology 507: 337–354.
Knorr-Cetina, K. (1999) Epistemic Cultures: How the Sciences Make Knowledge. Cambridge, MA: Harvard University Press.
Koenig, B. (1988) The technological imperative in medical practice: The social creation of a ‘routine’ treatment. In: M. Lock and R. Gordon (eds.) Biomedicine Examined. Dordrecht, The Netherlands: Kluwer Academic Publishers.
Kohn, D.B. and Gänsbacher, D. (2003) Letter to the editors of Nature from the American Society of Gene Therapy and the European Society of Gene Therapy. Journal of Gene Medicine 5(7): 641.
Landecker, H. (2007) Culturing Life: How Cells Became Technologies. Cambridge, MA: Harvard University Press.
Latour, B. (1987) Science in Action. Cambridge, MA: Harvard University Press.
Lehrman, S. (1995) … but seeks ‘practical’ gene therapy deals. Nature 378(6552): 7.
Liang, P. et al (2015) CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein and Cell 6(5): 363–372.
Lindee, S. (2005) Moments of Truth in Genetic Medicine. Baltimore, MD: John Hopkins University Press.
Lippman, A. (1992) Led astray by genetic maps: The cartography of the human genome project and healthcare. Social Science and Medicine 39(12): 1469–1476.
Lu, D.R. et al (1993) Stage 1 clinical trial of gene therapy for haemophilia B. Science in China 36(11): 1342–1351.
Lock, M. (2005) Eclipse of the gene and the return of divination. Current Anthropology 46(S48): S47–S70.
Lock, M. (2008) Biomedical technologies, cultural horizons and contested boundaries. In: E.J. Hackett, O. Amsterdamska, M. Lynch and J. Wajcman (eds.) Handbook of Science and Technology Studies, Third Edition. Cambridge, MA; London: MIT Press.
Lock, M. (2015) Comprehending the body in the era of the epigenome. Current Anthropology 56(2): 151–177.
Marshall, E. (1996) Gene therapy’s growing pains. Science 269(5227): 1050–1055.
Martin, P. (1999) Genes as drugs: The social shaping of gene therapy and the reconstruction of genetic disease. Sociology of Health and Illness 21(5): 517–538.
Miller, H. (1995) Overregulation is an unnecessary hindrance to human gene therapy. Human Gene Therapy 6(11): 1361–1362.
Miller, D.A. (1992) Human gene therapy comes of age. Nature 357(6368): 455–460.
Mitchell, R. and Waldby, C. (2010) National biobanks: Clinical labour, risk production, and the creation of biovalue. Science, Technology and Human Values 35(3): 330–355.
Nelkin, D. and Lindee, S. (1995) The DNA Mystique: The Gene as Cultural Icon. New York: W. H. Freeman.
NIH (2013) NIH guidelines for research involving recombinant or synthetic nucleic acid molecules, http://oba.od.nih.gov, accessed November 2013.
Novas, C. and Rose, N. (2000) Genetic risk and the birth of the somatic individual. Economy and Society 29(4): 485–513.
O’Reilly, M. et al (2013) NIH oversight of human gene transfer research involving retroviral, lentiviral, and adeno-associated virus vectors and the role of the NIH Recombinant DNA Advisory Committee. Methods in Enzymology 507: 313–335.
Orkin, S. and Motulsky, A. (1995) Report and recommendations of the panel to assess the NIH investment in research on gene therapy. Bulletin of Medical Ethics 116: 10–11.
President’s Commission for the Study of Ethical Problems in Medicine and Biomedical and Behavioral Research (1982) Splicing Life: A. Report on the Social and Ethical Issues of Genetic Engineering with Human Beings. Washington DC: U.S. Government Printing Office.
Rabeharisoa, V. and Callon, M. (2002) The Involvement of Patients’ Associations in Research. UNESCO report, Oxford, UK and Malden, MA.
Rabinow, P. (1996) Making PCR: A Story of Biotechnology. Chicago, IL; London: The University of Chicago Press.
Randall, C., Mandelbaum, B. and Kelly, T. (1980) Letter from three general secretaries. Letter to congress, Washington, DC.
Raper, S.E. et al (2003) Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Molecular Genetics and Metabolism 80(1–2): 148–158.
Rapp, R. (1999) Testing Women, Testing the Fetus: Women and Prenatal Diagnosis. New York: Routledge.
Schmek, H.M. (1981) U.S. agency disciplines gene therapy researcher. The New York Times, http://www.nytimes.com/1981/05/29/us/us-agency-disciplines-gene-splicing-researcher.html.
Sheridan, C. (2011) Gene therapy finds its niche. Nature Biotechnology 29(2): 121–128.
Stockdale, A. (1999) Waiting for the cure: Mapping the social relations of human gene therapy research. Sociology of Health and Illness 21(5): 579–596.
Stolberg, S.G. (1999) The biotech death of Jesse Gelsinger. The New York Times Magazine, 28 November, http://www.nytimes.com/1999/11/28/magazine/the-biotech-death-of-jesse-gelsinger.html?pagewanted=all.
Taussig, K.-S. (2009) Ordinary Genomes: Science, Citizenship, and Genetic Identities?. Durham, NC: Duke University Press.
Thompson, C. (2005) Making Parents: The Ontological Choreography of Reproductive Technologies. Cambridge, MA: MIT Press.
Uniqure (2012) uniQure’s Glybera® first gene therapy approved by European Commission. Press release. Amsterdam, The Netherlands.
Wade, N. (1981) Gene therapy caught in more entanglements. Science 212(4490): 24–25.
Wadman, M. (1995) Hyping results ‘could damage’ gene therapy. Nature 378(6558): 655.
Wailoo, K. and Pemberton, S. (2006) The Troubled Dream of Genetic Medicine: Ethnicity and Innovation in Tay-Sachs, Cystic Fibrosis, and Sickle Cell Disease. Baltimore, MD: John Hopkins University Press.
Wainwright, S.P., Williams, C., Michael, M., Farsides, B. and Cribb, A. (2006) Ethical boundary work in the embryonic stem cell laboratory. Sociology of Health and Illness 28(6): 732–748.
Wilson, J. (2005) Gendicine: The first commercial gene therapy product. Human Gene Therapy 16(9): 1014.
Wilson, J.M. (2009) Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Molecular Genetics and Metabolism 96(4): 151–157.
Wilson, J.M. (2013) Bulls, bubbles, and biotech. Human Gene Therapy 24(7): 715–716.
Wolff, J.A. and Lederberg, J. (1994) An early history of gene transfer and therapy. Human Gene Therapy 5(4): 469–480.
Yarborough, M. and Sharp, R.R. (2009) Public trust and research a decade later: What have we learned since Jesse Gelsinger’s death? Molecular Genetics and Metabolism 97(1): 4–5.
Ylä-Herttuala, S. (2012) Endgame: Glybera finally recommended for approval as the first gene therapy drug in the European Union. Molecular Therapy 20(10): 1831–1832.
Zallen, D.T. (2000) U.S. gene therapy in crisis. Genetics and Society 16(6): 272–275.
Acknowledgements
This article benefited greatly from feedback offered by Jesper Lassen, Peter Sandøe, Samuel Taylor-Alexander and the Work in Progress group led by Silvia Camporesi at KCL’s Department of Social Science Health and Medicine. This project, as part of the Consortium for Designer Organisms, is funded by the University of Copenhagen’s Excellence Fund for Interdisciplinary Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Addison, C. Spliced: Boundary-work and the establishment of human gene therapy. BioSocieties 12, 257–281 (2017). https://doi.org/10.1057/biosoc.2016.9
Published:
Issue Date:
DOI: https://doi.org/10.1057/biosoc.2016.9