Abstract
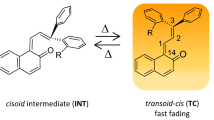
A novel biphotochromic compound (BPC) with two photochromic fragments, namely spironaphthopyran and hydroxyazomethine, was synthesized and studied by nanosecond laser flash photolysis using the excitation wavelengths of 337, 430, 470 and 500 nm in methanol and toluene. The photoexcitation of BPC results in the formation of at least two colored transients. The first one is the merocyanine form B (the maximum in the absorption spectrum is near 600 nm and the lifetime is 0.1 and 0.05 s in methanol and toluene, respectively) due to the spiro-bond break followed by isomerization. The second one is the trans-keto form AKt (the maximum in the absorption spectra is near 480 nm and the lifetime is 0.1 and 5 ms in methanol and toluene, respectively) as a result of cis-enol or cis-keto tautomer transformations. The relative yields of B and AKt depends essentially on the wavelength of excitation. The form AKt is the key transient formed under excitation with the visible light, whereas its yield under excitation with UV light is comparable with that of B. The specific solvation by methanol molecules favors the spirocycle opening even under visible light excitation. The results obtained for novel BPC were compared with those for other BPC where the same fragments are combined in such a way that the form B is the major one under excitation with UV light whereas it virtually is not observed under visible light excitation. The difference in both BPC is discussed in terms of conjugation (π-coupling) between photochromic fragments.
Similar content being viewed by others

References
V. I. Minkin, Bistable organic, organometallic, and coordination compounds for molecular electronics andspintronics, Russ. Chem. Bull., 2008, 57, 673–703.
A. P. De Silva, N. D. Mc Clenaghan, Molecular- scale logic gates, Chem.–Eur. J., 2004, 10, 574–586.
A. Fihey, A. Perrier, W. R. Browne and D. Jacquemin, Multiphotochromic molecular systems, Chem. Soc. Rev., 2015, 44, 3719–3759.
K. Higashiguchi, K. Matsuda, N. Tanifuji and M. Irie, Full-color photochromism of a fused dithienylethene trimer, J. Am. Chem. Soc., 2005, 127, 8922–8923.
S. Delbaere, G. Vermeersch, M. Frigoli and G. H. Mehl, Bridging the visible: the modulation of the absorption by more than 450 nm, Org. Lett., 2010, 12, 4090–4093.
J. Andreґasson, S. D. Straight, T. A. Moore, A. L. Moort and D. Gust, Molecular all-photonic encoder-decoder, J. Am. Chem. Soc., 2008, 130, 11122–11128.
J. Andreґasson, U. Pischel, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, All- photonics multifunctional molecular logic device, J. Am. Chem. Soc., 2011, 133, 11641–11648.
G. Sevez, J. Gan, S. Delbaere, G. Vermeersch, L. Sanguinet, E. Levillain, J.-L. Pozzo, Photochromic performance of a dithienylethene-indolinooxazolidine hybrid, Photochem. Photobiol. Sci., 2010, 9, 131–135.
A. Samat, V. Lokshin, K. Chamontin, D. Levi, G. Pepe and R. Guglielmetti, Spirooxazine photoisomerization and relaxation in polymer matrixes, Tetrahedron, 2001, 57, 7349–7359.
J. Berthet, J.-C. Micheau, A. Metelitsa, G. Vermeersch and S. Delbaere, Multistep Thermal Relaxation of Photoisomers in Polyphotochromic Molecules, J. Phys. Chem. A, 2004, 108, 10934–10940.
M. Frigoly and G. H. Mehl, Multiplrid biphotochromic system, Angew. Chem., Int. Ed., 2005, 44, 5048–5052.
K. Szacilowski, Digital information processing in molecular systems, Chem. Rev., 2008, 108, 3481–3548.
P. P. Levin, A. S. Tatikolov, N. L. Zaichenko, A. I. Shienok, L. S. Koltsova, O. Yu. Oskina, I. R. Mardaleishvili, L. D. Popov, S. I. Levchenkov and A. A. Berlin, Kinetics of photochemical reactions of multifunctional hybrid compounds based on spironaphthoxazines upon photoexcitation with light of different wavelength, J. Photochem. Photobiol., A, 2013, 251, 141–147.
N. L. Zaichenko, A. I. Shienok, L. S. Koltsova, I. R. Mardaleishvili, A. S. Tatikolov, P. P. Levin and A. A. Berlin, Bifunctional photosensitive compound for optical procrssrs, Phys. Status Solidi A, 2010, 2746–2748.
V. A. Nadtochenko, P. P. Levin, N. L. Zaichenko, F. E. Gostev, I. V. Shelaev, A. I. Shienok, L. S. Koltsova, O. M. Sarkisov and A. A. Berlin, Spectral and kinetic parameters of transient species in the photolysis of naphthylmethylideneiminospironaphthopyran by excitation at different wavelengths: nano_ and femtosecond laser photolysis, High Energy Chem., 2013, 47, 121–128.
H. Houjou, T. Motoyama, S. Banno, I. Yoshikawa, K. Araki, J. Org. Chem., 2000, 974, 520.
N. L. Zaichenko, P. P. Levin, I. R. Mardaleishvili, A. I. Shienok, L. S. Koltsova, O. Yu. Oskina and A. S. Tatikolov, Synthesis and kinetics of photochemical reactions of novel bifunctional salicylideneiminospironaphthoxazines, Russ. Chem. Bull., 2008, 57, 2394–2401.
P. P. Levin, N. L. Zaichenko, A. I. Shienok, L. S. Koltsova, I. R. Mardaleishvili and A. S. Tatikolov, Spectral kinetic characteristic of the photoisomerization products of naphthylmethylideneiminospironaphthopyran undused by photolysis at different wavelength, Russ. Chem. Bull., 2012, 61, 532–538.
Photochromism: Molecules and Systems, ed. H. Dürr and H. Bouas-Laurent, Elsevier, London, 2003, 1218 pp.
M. Z. Zgiersk and A. Grabowska, Photochromism of salicylideneaniline, J. Chem. Phys., 2000, 112, 6329–6337.
W. M. F. Fabian, L. Antonov, D. Nedeltcheva, F. S. Kamounah and P. J. Taylor, Tautomerism in Hydroxynaphthaldehyde Anils and Azo Analogues: a Combined Experimental and Computational Study, J. Chem. Phys. A, 108, 2004, 7603–7612.
M. Ziółek, J. Kubicki, A. Maciejewski, R. Naskrecki and A. Grabowska, Enol–keto tautomerism of aromatic photochromic Schiff base N,N′-bissalicylidene-p-phenylenediamine: Ground state equilibrium and excited state deactivation studied by solvatochromic measurements on ultrafast time scale, J. Chem. Phys., 2006, 124, 124518.
P. Fita, E. Luzina, T. Dziembowska, Cz. Radzewicz and A. Grabowska, Chemistry, photophysics, and ultrafast kinetics of two structurally related Shiff bases containing the naphthalene or quimoline ring, J. Chem. Phys., 2006, 125, 184508.
M. Ziołek, J. Kubicki, A. Maciejewski, R. Naskrecki, W. Luniewska and A. Grabowska, Unusual conformational effects in proton transfer kinetics of an excited photochromic Schiff base, J. Photochem. Photobiol., A, 2006, 180, 101–108.
M. Ziółek, M. Gil, J. A. Organero and A. Douhal, What is the difference between the dynamics of anion- and keto- type of photochromic salicylaldehyde azine?, Phys. Chem. Chem. Phys., 2010, 12, 2107–2115.
R. S. Becker and W. F. Richey, Photochromic aniles. Mechanism and products of photoreactions and thermal reactions, J. Am. Chem. Soc., 1967, 89, 1298.
S. Mitra and N. Tamai, Femtosecond spectroscopic study on photochromic salixylidene anilinem, Chem. Phys. Lett., 1998, 282, 391–397.
S. Mitra and N. Tamai, Dynamics of photochromism in salicylideneanilin: a femtosecond spectroscopic study, Phys. Chem. Chem. Phys., 2003, 5, 4647–4652.
C. Okabe, T. Nakabayashi, Y. Inokuchi, N. Nishi and H. Sekiya, Ultrafast excited-state dynamics in photochromic N-salicylideneaniline studied by femtosecond time-resolved REMPI spectroscopy, J. Chem. Phys., 2004, 121, 9436–9442.
M. Ziółek, J. Kubicki, A. Maciejewski, R. Naskręcki and A. Grabowska, Proton transfer reaction and photochromism of the new family of Schiff bases, Phys. Chem. Chem. Phys., 2004, 6, 4682–4689.
M. Sliwa, N. Mouton, C. Ruckebusch, L. Poisson, A. Idrissi, S. Aloise, L. Potier, J. Dubois, O. Poizat and G. Buntinx, Investigation of ultrafast photoinduced processes for salicylidene aniline in solutions and gas phase: towards a general photo-dynamic scheme, Photochem. Photobiol. Sci., 2010, 9, 661–669.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Levin, P.P., Tatikolov, A.S., Zaichenko, N.L. et al. Kinetics of photochemical reactions of biphotochromic compounds based on spironaphthopyran and enamine — conjugation effect. Photochem Photobiol Sci 15, 382–388 (2016). https://doi.org/10.1039/c5pp00314h
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c5pp00314h