Abstract
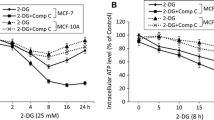
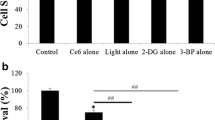
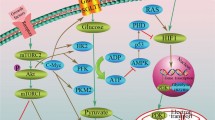
Malignant cells are highly dependent on aerobic glycolysis, which differs significantly from normal cells (the Warburg effect). Interference of this metabolic process has been considered as an innovative method for developing selective cancer therapy. A recent study demonstrated that the glycolysis inhibitor 2-deoxyglucose (2-DG) can potentiate PDT efficacy, whereas the possible mechanisms have not been carefully investigated. This study firstly proved the general potentiation of PDT efficacy by 2-DG and 3-bromopyruvate (3-BP) in human breast cancer MDA-MB-231 cells, and carefully elucidated the underlying mechanism in the process. Our results showed that both 2-DG and 3-BP could significantly promote a PDT-induced cell cytotoxic effect when compared with either monotherapy. Synergistic potentiation of mitochondria- and caspase-dependent cell apoptosis was observed, including a mitochondrial membrane potential (MMP) drop, Bax translocation, and caspase-3 activation. Besides, ROS generation and the expression of oxidative stress related proteins such as P38 MAPK phosphorylation and JNK phosphorylation were notably increased after the combined treatments. Moreover, when pretreated with the ROS scavenger N-acetylcysteine (NAC), the ROS generation, the MMP drop, cell apoptosis and cytotoxicity were differently inhibited, suggesting that ROS was vertical in the pro-apoptotic process induced by 2-DG/3-BP combined with PDT treatment. These results indicate that the combination of glycolytic antagonists and PDT may be a promising therapeutic strategy to effectively kill cancer cells.
Similar content being viewed by others
References
N. Hammoudi, K. B. Ahmed, C. Garcia-Prieto and P. Huang, The Warburg effect and its cancer therapeutic implications, Chin. J. Cancer, 2011, 30, 508–525.
M. G. Vander Heiden, L. C. Cantley and C. B. Thompson, Understanding the Warburg effect: the metabolic requirements of cell proliferation, Science, 2009, 324, 1029–1033.
L. Galluzzi, O. Kepp, M. G. Vander Heiden and G. Kroemer, Metabolic targets for cancer therapy, Nat. Rev. Drug Discovery, 2013, 12, 829–846.
C. Granchi and F. Minutolo, Anticancer Agents That Counteract Tumor Glycolysis, ChemMedChem, 2012, 7, 1318–1350.
T. N. Seyfried, R. E. Flores, A. M. Poff, D. P. D’Agostino, Cancer as a Metabolic Disease: Implications for Novel Therapeutics, Carcinogenesis, 2014, 35 3, 515–527.
L. P. Peter, Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen, J. Bioenerg. Biomembr., 2007, 39, 211–222.
P. M. Saroj, H. K. Young and L. P. Peter, Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg effect” and a pivotal target for effective therapy, Semin. Cancer Biol., 2009, 19, 17–24.
E. Hulleman, K. M. Kazemier, A. Holleman, D. J. VanderWeele, C. M. Rudin, M. J. Broekhuis, W. E. Evans, R. Pieters, M. L. Den Boer, Inhibition of glycolysis modulates prednisolone resistance in acute lympho-blastic leukemia cells, Blood, 2009, 113, 2014–2021.
D. Trachootham, J. Alexandre and P. Huang, Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?, Nat. Rev. Drug Discovery, 2009, 8, 579–591.
B. S. Dwarakanath, D. Singh, A. K. Banerji, R. Sarin, N. K. Venkataramana, R. Jalali, P. N. Vishwanath, B. K. Mohanti, R. P. Tripathi, V. K. Kalia and V. Jain, Clinical studies for improving radiotherapy with 2-deoxy-D-glucose: present status and future prospects, J. Cancer Res. Ther., 2009, 1, S21–S26.
H. Pelicano and D. S. Martin, R.-H. Xu and P. Huang, Glycolysis inhibition for anticancer treatment, Oncogene, 2006, 25, 4633–4646.
G. Maschek, N. Savaraj, W. Priebe, P. Braunschweiger, K. Hamilton, G. F. Tidmarsh, L. R. De Young and T. J. Lampidis, 2-Deoxy-D-glucose increases the efficacy of adriamycin and paclitaxel in human osteosarcoma and non-small cell lung cancers in vivo, Cancer Res., 2004, 64, 31–34.
L. S. Ihrlund, E. Hernlund, O. Khan and M. C. Shoshan, 3-Bromopyruvate as inhibitor of tumour cell energy metabolism and chemopotentiator of platinum drugs, Mol. Oncol., 2008, 2, 94–101.
P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Photodynamic therapy of cancer: An update, Ca-Cancer J. Clin., 2011, 61, 250–281.
S. Anand, B. J. Ortel, S. P. Pereira, T. Hasan and E. V. Maytin, Biomodulatory approaches to photodynamic therapy for solid tumors, Cancer Lett., 2012, 326, 8–16.
S. G. Bown, How mainstream medicine sees photodynamic therapy in the United Kingdom, J. Natl. Compr. Cancer Network, 2012, 10, S69–S74.
L. M. Davids and B. Kleemann, Combating melanoma: The use of photodynamic therapy as a novel, adjuvant therapeutic tool, Cancer Treat. Rev., 2011, 37, 465–475.
E. Crescenzi, A. Chiaviello and G. Canti, Low doses of cisplatin or gemcitabine plus Photofrin/photodynamic therapy: Disjointed cell cycle phase-related activity accounts for synergistic outcome in metastatic non-small cell lung cancer cells (H1299), Mol. Cancer Ther., 2006, 5, 776–785.
J. P. Golding, T. Wardhaugh, L. Patrick, M. Yurner, J. B. Phillips, J. I. Bruce and S. G. Kimani, Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy, Br. J. Cancer, 2013, 109 4, 976–982.
Z. J. Jin’s, About the evaluation of drug combination, Acta Pharmacol. Sin., 2004, 25, 146–147.
X. Wang, Q. Liu, P. Wang, Z. Wang, W. Tong, B. Zhu, Y. Wang and C. Li, Comparisons among sensitivities of different tumor cells to focused ultrasoundin vitro, Ultrasonics, 2009, 49, 558e564.
Y. Li, P. Wang, P. Zhao, S. Zhu, X. Wang and Q. Liu, Apoptosis induced by sonodynamic treatment by protoporphyrin IX on MDA-MB-231 cells, Ultrasonics, 2012, 52, 490–496.
D. Kessel, J. Reiners Jr., Light-Activated Pharmaceuticals: Mechanisms and Detection, Isr. J. Chem., 2012, 52, 674–680.
B. Wang, J. Wang, Q. Liu, H. Huang, M. Chen, K. Li, C. Li, X. Yu and P. Chu, Rose-bengal-conjugated gold nanorods for in vivo photodynamic and photothermal oral cancer therapies, Biomaterials, 2014, 35, 1954–1966.
N. Rubio, J. Verrax, M. Dewaele, T. Verfaillie, T. Johansen, J. Piette and P. Agostinis, p38(MAPK)-regulated induction of p62 and NBR1 after photodynamic therapy promotes autophagic clearance of ubiquitin aggregates and reduces reactive oxygen species levels by supporting Nrf2-antioxidant signaling, Free Radicals Biol. Med., 2014, 67, 292–303.
C. Kim, C. Chung, K. Choi, J. Yoo, D. Kim, Y. Jeong and D. Kang, Effect of 5-aminolevulinic acid-based photodynamic therapy via reactive oxygen species in human cholangiocarcinoma cells, Int. J. Nanomedicine, 2011, 6, 1357–1363.
P. He, J. Ahn, I. Shin and P. Chung, Photoactivation of 9-hydroxypheophorbide alpha triggers apoptosis through the reactive oxygen species-mediated mitochondrial pathway and endoplasmic reticulum stress in AMC-HN-3 laryngeal cancer cells, Int. J. Oncol., 2010, 36, 801–808.
H. Pelicano, D. S. Martin, R. H. Xu and P. Huang, Glycolysis inhibition for anticancer treatment, Oncogene, 2006, 25, 4633–4646.
M. Cuperlovic-Culf, A. Culf, M. Touaibia and N. Lefort, Targeting the latest hallmark of cancer: another attempt at ‘magic bullet’ drugs targeting cancers’ metabolic phenotype, Future Oncol., 2012, l8, 1315–1330.
S. Rello, J. C. Stockert, V. Moreno, A. Gámez, M. Pacheco, A. Juarranz, M. Cañete and A. Villanueva, Morphological criteria to distinguish cell death induced by apoptotic and necrotic treatments, Apoptosis, 2005, 10, 201–208.
H. P. Wang, X. B. Wang, P. Wang, K. Zhang, S. Yang and Q. H. Liu, Ultrasound enhances the efficacy of Chlorin e6-mediated photodynamic therapy in MDA-MB-231 cells, Ultrasound Med. Biol., 2013, 19, 1713–1724.
K. K. Arora and P. L. Pedersen, Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP, J. Biol. Chem., 1988, 263, 17422–17428.
A. M. Petros, A. Medek, D. G. Nettesheim, D. H. Kim, H. S. Yoon, K. Swift, E. D. Matayoshi, T. Oltersdorf and S. W. Fesik, Solution structure of the antiapoptotic protein BCL-2, Proc. Natl. Acad. Sci. U. S. A., 2001, 98 6, 3012–3017.
T. Kuwana, M. R. Mackey, G. Perkins, M. H. Ellisman, M. Latterich, R. Schneiter, D. R. Green and D. D. Newmeyer, BAX and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane, Cell, 2002, 111 3, 331–342.
A. G. Porter and R. U. Janicke, Emerging roles of caspase-3 in apoptosis, Cell Death Differ., 1999, 6 2, 99–104.
R. Hilf, R. S. Murant, U. Narayanan and S. L. Gibson, Relationship of Mitochondrial Function and Cellular Adenosine Triphosphate Levels to Hematoporphyrin Derivative-induced Photosensitization in R3230AC Mammary Tumors, Cancer Res., 1986, 46, 211–217.
K. Tobias, P. Kristjan, B. O. Christian, B. Juergen, J. O. Franz and K. Barbara, Differential effects of glucose deprivation on the cellular sensitivity towards photodynamic treatment-based production of reactive oxygen species and apoptosis-induction, FEBS Lett., 2005, 579, 185–190.
A. Matsuzawa and H. Ichijo, Stress-responsive protein kinases in redox-regulated apoptosis signaling, Antioxid. Redox Signaling, 2005, 7, 472–481.
V. Temkin and M. Karin, From death receptor to reactive oxygen species and c-Jun N-terminal protein kinase: The receptor-interacting protein 1 odyssey, Immunol. Rev., 2007, 220, 8–21.
Y. M. Ha, M. K. Park, H. J. Kim, H. G. Seo, J. H. Lee and K. C. Chang, High concentrations of ascorbic acid induces apoptosis of human gastric cancer cell by p38-MAP kinase-dependent up-regulation of transferrin receptor, Cancer Lett., 2009, 277, 48–54.
A. Vibhuti, K. Muralidhar and B. S. Dwarakanath, Differential cytotoxicity of the glycolytic inhibitor 2-deoxy-D-glucose in isogenic cell lines varying in their p53 status, J. Cancer Res. Ther., 2013, 9 4, 686–692.
V. Jain, Modifications of radiation responses by 2-deoxy-D-glucose in normal and cancer cells, Indian J. Nucl. Med., 1996, 11, 8–17.
H. Xi, J. C. Barredo, J. R. Merchan and T. J. Lampidis, Endoplasmic reticulum stress induced by 2-deoxyglucose but not glucose starvation activates AMPK through CaMKKβ leading to autophagy, Biochem. Pharmacol., 2013, 85 10, 1463–1477.
Q. Wang, B. Liang, N. A. Shirwany and M. H. Zou, 2-Deoxy-D-glucose treatment of endothelial cells induces autophagy by reactive oxygen species-mediated activation of the AMP-activated protein kinase, PLoS One, 2011, 6 2, e17234.
C. Rodrigues-Ferreira, A. P. da Silva and A. Galina, Effect of the antitumoral alkylating agent 3-bromopyruvate on mitochondrial respiration: role of mitochondrially bound hexokinase, J. Bioenerg. Biomembr., 2012, 44 1, 39–49.
S. Ganapathy-Kanniappan, M. Vali, R. Kunjithapatham, M. Buijs, L. H. Syed, P. P. Rao, S. Ota, B. K. Kwak, R. Loffroy and J. F. Geschwind, 3-bromopyruvate: a new targeted antiglycolytic agent and a promise for cancer therapy, Curr. Pharm. Biotechnol., 2010, 11 5, 510–517.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, X., Zhang, Y., Wang, P. et al. Energy metabolism targeted drugs synergize with photodynamic therapy to potentiate breast cancer cell death. Photochem Photobiol Sci 13, 1793–1803 (2014). https://doi.org/10.1039/c4pp00288a
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c4pp00288a