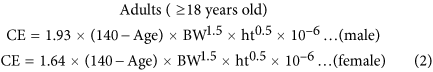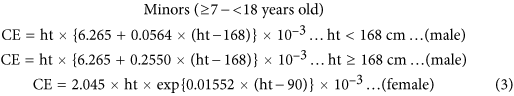Abstract
A food scandal occurred in Taiwan in 2011 because the DEHP (di-2-ethylhexyl phthalate) had been intentionally used in food products. We assessed the daily intakes (DIs) and cumulative risk of phthalates in Taiwan’s general population after the scandal. The DIs of 6 phthalates, including di-n-butyl phthalate (DnBP), di-iso-butyl phthalate (DiBP), and DEHP, were evaluated using urinary phthalate metabolites. Hazard quotients of phthalates classified as affecting the reproductive (HQrep) and hepatic (HQhep) systems were assessed using cumulative approach. The creatinine-based model showed that the highest DI values in children 7-to 12- years-old were for DEHP (males: median: 4.79 μg/kg bw/d; females: median: 2.62 μg/kg bw/d). The 95th percentile (P95) of HQrep values were all >1 in the 7- to 12-year-old and 18- to 40-year-old male groups. The P95 of HQhep values were all >1 in the 7- to 18- year-old male groups. Most of the HQrep was attributable to the HQs of DnBP and DiBP (53.9–84.7%), and DEHP contributed most to HQhep (83.1–98.6%), which reveals that DnBP, DiBP and DEHP were the main risk of phthalate exposure for Taiwanese. Taiwan’s general population is widely exposed to DnBP, DiBP and DEHP, especially for young children.
Similar content being viewed by others
Introduction
Phthalates are synthetic aromatic chemicals adding in a variety of products used in everyday life1,2. Di-(2-ethylhexyl) phthalate (DEHP), di-n-octyl phthalate (DnOP), di-isononyl phthalate (DiNP), and diisodecyl phthalate (DiDP) belongs to the high-molecular-weight phthalates and are used as plasticizers in building materials and furniture, and di-n-butyl phthalate (DnBP), di-i-butyl phthalate (DiBP), dimethyl phthalate (DMP). and diethyl phthalate (DEP) are used in personal-care products (e.g., nail polish, fragrance, etc.), lacquers, varnishes, and coatings for their nature of low molecular weight3. The major phthalates exposure route for the general population are ingestion, inhalation, and dermal absorption4,5,6. Dietary exposure is believed to be the major important source for the general population7,8,9. Additionally, young children could be exposed to phthalates when they swallow or inhale dust while playing on the floor, and by chewing PVC containing toys and products10,11. The phthalate metabolites have been measured in a representative U.S. general population urinary samples12, in some European countries13,14,15 and Asian countries16,17 have also been reported, which indicates that phthalates exposure can happen anywhere, at any time.
Based on human-biomonitoring data, the risk assessment of different phthalates can be individually evaluated. Human-biomonitoring is a method used to approximate the total background exposure of phthalic acid esters (PAEs). Furthermore, based on the hazard index (HI) approach18,19, which assumes dose addition20,21, a cumulative risk assessment for various exposures to chemicals with similar toxicity has been developed for PAEs. As outlined in a National Research Council (NRC) report (2008), the HI provides a direct and simple method for relating the intake of a certain of chemicals to their reference values (RfVs). Examples of RfVs for oral exposure include the U.S. EPA reference doses (RfDs)21, and the European Food Safety Authority (EFSA) tolerable daily intake (TDI)22,23,24,25. The hazard quotient (HQ) is estimated as the ratio of the calculating exposure level to the RfV of corresponding chemical. The HQs are then added together to estimate the overall HI.
After the 2011 Taiwan DEHP scandal, the Taiwan Food and Drug Administration (TFDA) adopted the EFSA TDI values. It is difficult to know whether the current reference values protect general Taiwanese. Several parameters of risk assessment of PAEs vary widely based on ethnicity26,27. Therefore, we used human-biomonitoring data from our published study to estimate the daily exposure dose and cumulative risk of phthalate in Taiwan’s general population. Additionally, we also try to identify potential differences and trends in exposure to phthalates. The objectives of this study were: (1) to estimate the daily intakes (DIs) of six phthalates based on their urinary levels; and (2) to assess the cumulative risk of exposure to phthalates based on anti-androgenic and hepatic endpoints in a Taiwanese general population.
Results
The detectable rate of phthalate metabolites was highest in MEHHP and lowest in MiNP in all urine samples. Geometric means were from ND to 32.7 mg/L for the 10 phthalate metabolites measured. The median level of MEP (creatinine-unadjusted) in the 18–40 years old group was significantly (p < 0.001) higher than that in the other age groups, especially for women. Additionally, median levels of MnBP and MiBP in all participants decreased along with increasing age. There was no significant change of MEHP in our participants regardless of age or gender.
Estimated daily intake of phthalates
The risks associated with exposure to phthalates were assessed based on the DI of each participant (Table 1). The DI was compared with the EFSA TDI acceptable exposure level in order to estimate potential exceedances. The DIs of DnBP, DiBP, and DEHP decreased with increasing age. Median DIs of DnBP, DiBP, and DEHP were nearly four times as high in the ≥7–<12 years old than in the >65 years old males group. The DIs of DiBP and DEHP for the ≥7–<12 years old females group were all slightly higher than those for the other female age groups. The DIs of DEHP for males were all slightly higher than those for females in all age groups. The creatinine-based calculation model showed that the highest DI values in males and females ≥7–<12 years old were for DEHP (males: median: 4.79 μg/kg bw/d; 95th percentile (P95): 22.6 μg/kg bw/d; females: median: 2.62 μg/kg bw/d; P95: 12.6 μg/kg bw/d). DEHP also had the highest DI values in young adult (≥18–<40 years old) males and females (males: median: 4.03 μg/kg bw/d; P95: 12.1 μg/kg bw/d; females: 2.31 μg/kg bw/d; P95: 18.2 μg/kg bw/d). DIs for BBzP and DiNP were considerably lower in all age-gender groups, but within the same range (median: 0. 005–0.252 μg/kg bw/day).
Reproductive and hepatic evaluation. Cumulative risk assessment: HQ and HI
More males had higher HQrep values: HQrep > 1: males = 2.66%; females = 2.01% (Fig. 1). The P95 of HQrep values were all >1 in the ≥7–<12-year-old, 18- to 40-year-old, and ≥65-year-old male groups, but they were <1 in all of the female groups. More males had higher HQhep values: HQhep > 1: males = 2.13%; females = 0.50%. The P95 of HQhep values were all > 1 in the ≥7–< 12-year-old and 12- to 18- year-old male groups, but they were <1 in all of the female groups.
Except for 18- to 40-year-olds, in the other age groups, HQhep values were non-significantly higher in males than in females; however, in the ≥40- to <65-year-old group, the difference was significant (p = 0.001) (Fig. 1). Although median values of HQhep were comparable in males and females, the P95 was about twice as high for males than for females in the ≥7–<12-year-old, ≥18- to <40-year-old, and >65-year-old age groups. Differences in gender were particularly pronounced in the ≥7–≤12 years old, and ≥12- to <18 years old groups. In addition, HQrep values in males were also slightly higher than in females, except for the ≥18- to <40-year-olds (Fig. 1). Although the median values of HQrep were comparable in males and females, the P95 was about twice as high for males than for females in the ≥7–<12 and >65 years old groups. Differences in gender were particularly pronounced in the children and in the elderly.
In general, most of the HQrep was attributable to the HQs of DnBP and DiBP (53.9–84.7%), and DnBP and DiBP combined was the main risk of phthalate exposure for Taiwanese adults (Fig. 2). DEHP contributed most to HQhep (83.1–98.6%), which might have been because of the higher DEHP exposure dose and hepatic toxicity than of the other two phthalates (Fig. 2).
Figure 3 illustrates the distribution plots of HQhep and HQrep (logarithmic scale for the x-axis) against the relative cumulative frequency distribution, which must be seen as a first crude approach for determining the cumulative exposure and additive toxicity of phthalates. Depending upon the cumulative model, 4 males (3 were <18 years old [5.6%]; 1 was ≥18 years old [0.75%]) and 1 female (≥18 years old [0.64%]) had high (>1) HQhep values. In addition, 5 males (1 was <18 years old [1.9%]; 4 were ≥18 years old [2.99%]) and 4 females (≥18 years old [2.56%]) had high (>1) HQrep values, which indicated a considerable level of cumulative exposure to phthalates for Taiwanese adults.
Principal Component Analysis (PCA)
To evaluate the exposure profile of phthalates in different age and gender, the PCA results are divided into male minors (<18 years old), male adults (≥18 years old), female minors, and female adults, and described in Fig. 4 and Supplementary Table 2. Three principal components were extracted from male minors, which accounted for 37.8% (Principal Component 1 [PC1]), 18.9% (PC2), and 12.0% (PC3) of the variability. This indicated three major potential sources of exposure to phthalates for Taiwanese male minors. The 5 DEHP metabolites—MEHP, MEHHP, MEOHP, MECPP, and MCMHP—were highly correlated with PC1, and MiBP and MnBP were highly correlated with PC2. Three principal components were extracted from male adults, which accounted for 36.6% (PC1), 13.4% (PC2), and 11.6% (PC3) of the variability. Four DEHP metabolites—MEHHP, MEOHP, MECPP, and MCMHP—were highly correlated with PC1, and MnBP and MiNP were highly correlated with PC2. Three principal components were extracted from female minors, which accounted for 33.9% (PC1), 14.7% (PC2), and 13.3% (PC3) of the variability. The 4 DEHP metabolites—MEHHP, MEOHP, MECPP, and MCMHP—were highly correlated with PC1, and MiBP and MiNP were highly correlated with PC2. Three principal components were extracted from female adults, which accounted for 39.2% (PC1), 17.9% (PC2), and 11.7% (PC3) of the variability. The 4 DEHP metabolites—MEHHP, MEOHP, MECPP, and MCMHP—were highly correlated with PC1, and MBzP and MiNP were highly correlated with PC2. In addition, MiBP and MnBP were highly correlated with PC3. Moreover, in male minors, our cluster analysis (CA) identified two major clusters of urinary metabolites: (1) the DEHP metabolites (MEHP, MEHHP, MEOHP, MECPP, and MCMHP); and (2) MiBP and MnBP (Fig. 5). In male adults, CA identified two major clusters of urinary metabolites: (1) the DEHP metabolites (MEHHP, MEOHP, MECPP, and MCMHP); and (2) MnBP and MiNP. In female minors, CA identified two major clusters of urinary metabolites: (1) the DEHP metabolites (MEHHP, MEOHP, MECPP, and MCMHP); and (2) MiBP and MiNP. In female adults, CA identified three major clusters of urinary metabolites: (1) the DEHP metabolites (MEHHP, MEOHP, MECPP, and MCMHP); (2) MBzP and MiNP; and (3) MiBP and MnBP.
We used information from the questionnaire to evaluate the relationships between urinary phthalate metabolites, phthalate DIs, and family members (Supplemental Table 3). The correlation coefficients (r) of urinary MiBP, MnBP, MEHHP, MEOHP, MECPP, MCMHP, and ΣDEHP were higher in couples and siblings than in parents. Moreover, the r of DiBP, DnBP, DEP, and ΣDEHP DIs were also higher in couples and siblings than in parents.
Discussion
In the present study, we systematically measured our study sample’s daily exposure to phthalates based on biomonitoring data, and provide a comprehensive and cumulative estimated risk of exposure to phthalates for Taiwan’s general population, the first study to do so.
Our human-biomonitoring data are measurements of internal doses from all routes (inhalation, dermal, and oral) and sources of exposure to phthalates, and provide an effective tool for assessing the general population’s exposure. Most assessments of exposure to phthalates in currently focused on human-biomonitoring2,28,29. The National Health and Nutrition Examination Survey (NHANES) data have been used to back-calculate daily exposure to chemicals in the US general population30,31. In a recent study, reverse dosimetry approach were used to reconstruct exposure from urinary concentrations of 82 NHANES chemicals involving phthalate compounds to prioritize their risk32. Hays and colleagues developed the biomonitoring equivalents to evaluate the risk from urine data for DiNP33. Several studies have reconstructed daily does from phthalate or its metabolites in urine for comparison to the existing RfD34,35. These studies show that exposure reconstruction from phthalate and its metabolites in urine are regarded as the most reliable method to quantify overall exposure to phthalates because phthalate metabolites in urine are not likely to have bias by external contamination.
The results generated by dose addition models were consistent with actual administration experiments of several phthalates simultaneously36,37,38,39,40. To protect the public’s health, the TDI was used to estimate the amount of a chemical in air, food, and drinking water that can be consumed everyday over a lifetime without appreciable health risk. In the present study, the maximum and P95 values for DnBP and DEHP in male minors group were very close the TDI reference values, which indicates that the latter for some individual PAEs are recommended to be modified. Therefore, we can also protect the most vulnerable segments of the general population from exposure to PAEs. In Taiwan, there is no suggested TDI value for DiBP, even though this phthalate was reported41 to reduce fetal testosterone production with a potency similar to that of both DnBP and DEHP, which suggests that it is appropriate to use the EFSA TDI value for DnBP to estimate exposure to DnBP and DiBP simultaneously. For vulnerable groups—especially children—the growing evidence indicated that growth and development of the reproductive and endocrine systems could be disrupted42,43,44,45. In addition, results from the recent toxicological phthalates studies have indicated a consideration of each TDI for each individual phthalate would be inappropriate for the overall tolerable phthalate intake38,39,40. The omnipresent exposure to a lot of phthalates and the understanding that these phthalates act in a dose-additive nature derived a cumulative risk assessment method38,39,40.
We used a set of equations developed by Mage et al.26,27 to predict our participants’ expected daily creatinine excretion (CE) (mg/kg) as a function of age, gender, and anthropometric measurements, in two age groups (≥7–18 and ≥18 years old). We also compared estimated DIs using the CE equations separately developed by Mage et al.26,27 and Kawasaki et al.46. Although the Mage et al. CE equation yields a lower DI, we still use it because it considers different physiological parameters for each age group, especially for minors. The HQ and HI approaches provide a forthward method to evaluate non-cancer risk for a given level of chemical exposure.
Calculating and interpreting the HQ and HI depend upon the method used to estimate level of exposure, and on the choice of a reference value. The creatinine correction approach evaluated by Aylward et al.47, assumes that a sampled concentration sufficiently represents a daily average concentration. Thus, DIs derived from spot samples might range widely of the actual DI (20% to 300%), although this variability might affect the accuracy of an estimated intake for a single individual. A group of spot urine samples provides a reasonable approximation of concentrations that could have been observed in a population of full-day urine samples collected from the same population for phthalates48,49.
Principal component analysis (PCA) was used to characterize the similarity of urinary phthalate metabolites in each participant. Cluster analysis yielded similar findings. We found that the primary component of exposure to PAEs in Taiwanese was DEHP, regardless of age or gender. Guo et al.50 found that DEHP was the most abundant phthalate in food in a Chinese population. Due to a similarity of life style and habit of food consumption in Han population, food might also be the major source of exposure to DEHP for the Taiwan general population. The secondary components of exposure to PAEs in Taiwanese were MiBP, MnBP, MBzP, and MiNP. The sources of DiBP, DnBP, BBzP, and DiNP were more complicated. Guo et al. and Guo and Kannan claimed that diet, dust, and personal care products were not major sources of exposure to DnBP and DiBP for the Chinese general population50,51,52. We previously found that beverages were the primary contributors (about 30–60%) in the overall estimates of average daily doses (ADDs) for all PAEs53. This might raise public concern, because the health of some Taiwanese (children in particular) is probably being negatively affected because they drink too much artificially sweetened tea and other soft drinks from phthalate-containing plastic cups and bottles. We also found that the P95 HIs of anti-androgenic phthalates in children were already >1.
Despite the claims of Guo et al. and Guo and Kannan50,51,52, others51,54,55 concluded that personal care products application were the major sources of DEP and DnBP in the environment. Parlett et al. and Philippat et al. documented that increased phthalate levels in women were correlated with using greater amounts of cosmetic, perfume, and personal care products56,57. We previously found that only 45% of Taiwanese women used lotion and body wash every day, and that less than 5% of them used perfume and nail polish frequently58. Therefore, personal care products might be another source of exposure to phthalates for the Taiwan general population. Moreover, the correlation between urinary phthalate metabolites and phthalate DIs of DiBP, DnBP, DEP, and ΣDEHP in couples and siblings are higher than in their parents. This probably indicates that cohabiting adults are more similar in their levels of exposure to PAE than are parents and the children they are raising, or that the older people become, the fewer phthalate-containing personal care products they use.
This study has several strengths. First, it is a nationwide sample with participants between 7 and 97 years old groups. These data provide reference values of phthalates in the Taiwanese general population. Second, we use an appropriate equation that estimates daily creatinine excretion (mg/day) based on several physiological parameters. This developed equation is piecewise continuous for males and females from 7 to 97 years old. Third, we determined the internal exposure to phthalates in the general population and used it to estimate phthalate-related health risks. This study has some limitations. First, the distribution of individual exposure to phthalates in a general Taiwanese population after a DEHP food scandal can vary and the cross-sectional study design with one-time-point measurements cannot sufficiently assess exposure over time. The estimation of phthalate DIs is based on urinary metabolite levels measured in first morning samples and on the premise that the concentrations of metabolites in these spot morning samples are representative of the daily average urinary levels. Second, we did not have sufficient evidence to link decreased phthalate exposure to this DEHP food scandal. Legal restrictions on the products in which phthalates can be used, and the permissible levels of phthalates might decrease phthalate exposure levels in the general population.
Conclusion
We assessed the DIs and cumulative risks of 6 phthalates in a Taiwan population based on the urinary phthalate metabolites after the 2011 DEHP food scandal. Our data indicated that the Taiwanese general population is still widely exposed to phthalates after restrictions and legislation on phthalates, and showed that HQhep and HQrep values were slightly higher in males than in females, especially for 18- to 40-year-olds. We also found two components of exposure to PAEs: the primary was DEHP and the secondary were DiBP, DnBP, and BBzP. Additional studies are needed to clarify whether the contamination sources primarily food and personal care products. We suggest that Taiwan’s government lower the TDI of DEHP to protect vulnerable residents (children and adults of childbearing age) after considering the potential cumulative negative effects on reproduction.
Materials and Methods
Study Participants and Biomonitoring Data of Phthalates in Human Urine Samples
The subjects recruiting and sampling process were approved by the Research Ethic Committee of National Health Research Institutes (No. EC1020206) and described elsewhere58. The methods were performed in accordance with the approved guidelines.
After a written informed consent on behalf of the participated children was obtained from their parents and each child, participants provided a first morning urine sample and filled in data on a systematic questionnaire about participant demographics (age, gender, body weight, body height, and residence). The participants recruited consisted of 199 females and 188 males living in 22 cities and counties in Taiwan. The participants were between 7 and 97 years old, with an average body mass index (BMI) of 23.5 kg/m2. Immediately after the May-to-December 2013 urine collection, the samples were aliquoted, stored at −80 °C and thawed at −20 °C before sample pretreatment. Therefore, the urinary levels of phthalate metabolites were measured in the following participants: children (≥7–<12 years old), adolescents (≥12–<18 years), young adults (≥18–40 years), middle-aged adults (≥40–<65 years), and the elderly (≥65 years) were analyzed and used to calculate DIs of PAEs and to estimate cumulative risks. To calculate the DIs of PAEs and then the hazard index (HI), which was recently established for assessing the cumulative risk of phthalates exposure19,59,60, we used the results of urinary levels of PAE metabolites determined in our previous study58. By considering the cumulative hazard of several phthalates with similar toxicity, this index was evaluated by adding the ratios between TDI and reference limits (TDI or RfD anti-androgenicity) for the different compounds. The first-morning urine samples were analyzed for ten phthalate metabolites [mono-ethylhexyl phthalate (MEHP), mono-(2-ethyl-5-oxo-hexyl) phthalate (MEOHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(2-carboxymethylhexyl) phthalate (MCMHP), mono-n-butyl phthalate (MnBP), mono-iso-butyl phthalate (MiBP), monoethyl phthalate (MEP), mono-iso-nonyl phthalate (MiNP), and mono-benzyl phthalate (MBzP)] which are biomarkers for exposure to the six commonly used phthalates [DEHP, DnBP, DiBP, DEP, DiNP, and benzyl butyl phthalate (BBzP)]. We used an online modified analytical method coupled to a liquid chromatograph/electrospray tandem mass spectrometer (Agilent 1200/API 4000; Applied Biosystems, Foster City, CA, USA) method discussed by Koch et al.61,62 with quantification by isotope dilution.
Calculating Daily Intakes (DIs)
To calculate the DIs of each phthalate, the urinary phthalate metabolite levels in the spot urine samples and the individual age, body weight (BW), and body height (ht) data of each participant were combined. The individual DIs of phthalates based on urinary phthalate metabolites were calculated using the method described by Koch et al.63:
- 1
UEsum is the molar urinary excretion sum of the measured urinary phthalate metabolites;
- 2
The smoothed creatinine excretion (CE) rates CEsmoothed are age, body weight (BW) and height (ht), and gender-based values for urinary CE26,27. The formulae of CEsmoothed estimates for adults and minors in this study are listed below:
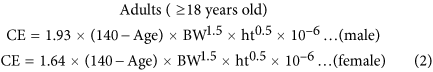
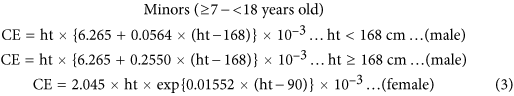
- 3
where Age (years old) and ht (cm) are the participant’s age and height, which were obtained from the questionnaire;
- 4
FUE, the molar fraction, describes the molar ratio between the excreted amounts of the specific metabolites of each phthalate corresponding to the dietary intake of the parent phthalate2,64.

All of the parameters we used for calculating DIs are listed in Supplemental Table 1.
Cumulative Risk Assessment — Hazard Quotient and Hazard Index
To assess the participant’s risk from each phthalate, we used the hazard quotient (HQ) as following formula:

Where HQ is the hazard quotient for an individual phthalate, the reference limit value (RLV) is the TDI or RfD. The RLV selected for DnBP, BBzP and DEHP by the EFSA22,23,24 were 10, 500 and 50 μg/kg-BW/day, and RfDs developed for BBzP and DEHP by the U.S. EPA65,66 were 200 and 20 μg/kg-BW/day. There was no TDI or RfD for DiBP; thus, the DnBP value was based on analogy assignment, 10 μg/kg-BW/day19,59. The TDI values of DnBP, DEHP, and BBzP set up by the EFSA were based on anti-androgenic effects (developmental and testicular toxicity) in animal models22,23,24.
An HI < 1 indicates no significant adverse effects from several chemicals exposure could happen67. Estimated HI values of cumulative hepatic effect were calculated based on the RfD (HQ of BBzP, DiNP, and DEHP).

Because not all of the U.S. EPA’s RfDs for phthalates were established based on anti-androgenic effects, HI values were estimated based only on the TDIs (HQ of DnBP, DiBP, BBzP, and DEHP). Although the TDI and RfD for DiBP could not be obtained, it was assuming DiBP with the values of DnBP for their similar structure and toxicity36. DEP is not included in the anti-androgenic assessment for its corresponding toxicity68.

We calculated the DIs for each phthalate and compare them to the EFSA’s TDIs; normalized each TDI to 100%; summed the DI percentages calculated in relation to the respective TDIs; and then checked to see whether the cumulative TDI (at 100%) had been exceeded.
Principle Component Analysis
We used principle component analysis (PCA) for 10 phthalate metabolites (MEP, MBzP, MnBP, MiBP, MiNP, and the five DEHP metabolites) to identify potential sources. The number of components to retain was based on score plot analysis and eigenvalue [1.1] criteria. We use varimax (orthogonal) rotation to obtain a set of independent interpretable factors according to a factor loading >0.55 (or <−0.55) with a particular factor are considered to be its major constituents. In addition, an agglomerative hierarchical clustering analysis was used to group the phthalate metabolites into clusters.
Statistical Analysis
We report phthalate results as μg/g of creatinine (μg/g Cr). Creatinine was used to adjust for individual variations in urine concentration. The non-detectable (ND) levels, i.e., those below the limit of detection, were calculated as half of the detection limit of each phthalate metabolite, and the detectable rate as the number of urine samples with the level of each phthalate metabolite above the detection limit, divided by all of the analyzed urine samples. We categorized our participants into five comparably sized age groups: ≥7–<12 years old, ≥12 to <18 years old, ≥18 to <40 years old, ≥40 to <65 years old, and ≥65 years. The Mann-Whitney U test was used to evaluate differences between demographic data, e.g., age and gender, and the Kruskal-Wallis test was used to evaluate differences between each level of phthalate metabolites. SPSS 22.0 (SAS Institute, Cary, NC, USA) for Windows was used for all statistical analyses. Significance was set at p < 0.05.
Additional Information
How to cite this article: Chang, J.-W. et al. Estimated Daily Intake and Cumulative Risk Assessment of Phthalates in the General Taiwanese after the 2011 DEHP Food Scandal. Sci. Rep. 7, 45009; doi: 10.1038/srep45009 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aylward, L. L., Kirman, C. R., Schoeny, R., Portier, C. J. & Hays, S. M. Evaluation of Biomonitoring Data from the CDC National Exposure Report in a Risk Assessment Context: Perspectives across Chemicals. Environ Health Perspect 121, 287–294 (2013).
Wittassek, M., Koch, H. M., Angerer, J. & Bruning, T. Assessing exposure to phthalates - The human biomonitoring approach. Mol Nutr Food Res 55, 7–31 (2011).
Schettler, T. Human exposure to phthalates via consumer products. Int J Androl 29, 134–139 (2006).
Gong, M. Y., Zhang, Y. P. & Weschler, C. J. Measurement of Phthalates in Skin Wipes: Estimating Exposure from Dermal Absorption. Environ Sci Technol 48, 7428–7435 (2014).
Hauser, R. & Calafat, A. M. Phthalates and human health. Occupational and Environmental Medicine 62 (2005).
Wormuth, M., Scheringer, M., Vollenweider, M. & Hungerbuhler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Analysis 26, 803–824 (2006).
European Chemicals Bureau. Risk assessment report for bis(2-ethylhexyl) phthalate (consolidated final report: February 2004). Doc. No. R042_0402_env_hh_4-6. Ispra, Italy. (2004).
Meek, M. E. & Chan, P. K. L. Bis(2-Ethylhexyl)Phthalate - Evaluation of Risks to Health from Environmental Exposure in Canada. Environmental Carcinogenesis & Ecotoxicology Reviews-Part C of Journal of Environmental Science and Health 12, 179–194 (1994).
Petersen, J. H. & Breindahl, T. Plasticizers in total diet samples, baby food and infant formulae. Food Addit Contam 17, 133–41 (2000).
Becker, K. et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health 207, 409–17 (2004).
Koch, H. M., Drexler, H. & Angerer, J. Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP). Int J Hyg Environ Health 207, 15–22 (2004).
Silva, M. J. et al. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect 112, 331–338 (2004).
Hogberg, J. et al. Phthalate diesters and their metabolites in human breast milk, blood or serum, and urine as biomarkers of exposure in vulnerable populations. Environ Health Perspect 116, 334–339 (2008).
Lopez-Carrillo, L. et al. Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environ Health Perspect 118, 539–544 (2010).
Ye, X. B. et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: The Generation R study. Environ Res 108, 260–267 (2008).
Itoh, H., Yoshida, K. & Masunaga, S. Quantitative identification of unknown exposure pathways of phthalates based on measuring their metaholites in human urine. Environ Sci Technol 41, 4542–4547 (2007).
Ji, K., Kho, Y. L., Park, Y. & Choi, K. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants. Environ Res 110, 375–382 (2010).
Kortenkamp, A. & Faust, M. Combined exposures to anti-androgenic chemicals: steps towards cumulative risk assessment. Int J Androl 33, 463–74 (2010).
Soeborg, T., Frederiksen, H. & Andersson, A. M. Cumulative risk assessment of phthalate exposure of Danish children and adolescents using the hazard index approach. Int J Androl 35, 245–52 (2012).
U.S. Environmental Protection Agency. Framework for Cumulative Risk Assessment. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington Office, Washington, DC. EPA/600/P-02/001F (2003).
U.S. Environmental Protection Agency. Concepts, Methods, and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects: A Resource Document (Final Report). U.S. Environmental Protection Agency, Washington, DC. EPA/600/R-06/013F (2007).
European Food Safety Authorities. Opinion of the scientific panel on food additives flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to di-butylphthalate (DBP) for use in food contact materials. EFSA J 242, 1–17 (2005a).
European Food Safety Authorities. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to butylbenzylphthalate (BBP) for use in food contact materials. EFSA J 241, 1–14 (2005b).
European Food Safety Authorities. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to bis(2-ethylhexyl)phthalate (DEHP) for use in food contact materials. EFSA J 243, 1–20 (2005c).
European Food Safety Authorities. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to di-isononylphthalate (DINP) for use in food contact materials. EFSA J 244, 1–18 (2005d).
Mage, D. T. et al. Estimating pesticide dose from urinary pesticide concentration data by creatinine correction in the Third National Health and Nutrition Examination Survey (NHANES-III). J Expo Anal Environ Epidemiol 14, 457–465 (2004).
Mage, D. T., Allen, R. H. & Kodali, A. Creatinine corrections for estimating children’s and adult’s pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. Journal of Exposure Science and Environmental Epidemiology 18, 360–368 (2008).
Koch, H. M. & Calafat, A. M. Human body burdens of chemicals used in plastic manufacture. Philosophical Transactions of the Royal Society B-Biological Sciences 364, 2063–2078 (2009).
Wang, W. et al. Size fraction effect on phthalate esters accumulation, bioaccessibility and in vitro cytotoxicity of indoor/outdoor dust, and risk assessment of human exposure. J Hazard Mater 261, 753–62 (2013).
Kohn, M. C. et al. Human exposure estimates for phthalates. Environ Health Perspect 108, A440–2 (2000).
David, R. M. Exposure to phthalate esters. Environ Health Perspect 108, A440–A440 (2000).
Wambaugh, J. F. et al. High-throughput models for exposure-based chemical prioritization in the ExpoCast project. Environ Sci Technol 47, 8479–88 (2013).
Hays, S. M., Aylward, L. L., Kirman, C. R., Krishnan, K. & Nong, A. Biomonitoring equivalents for di-isononyl phthalate (DINP). Regul Toxicol Pharmacol 60, 181–8 (2011).
Dewalque, L., Charlier, C. & Pirard, C. Estimated daily intake and cumulative risk assessment of phthalate diesters in a Belgian general population. Toxicol Lett 231, 161–168 (2014).
Gao, C. J. et al. Phthalate metabolites in urine of Chinese young adults: Concentration, profile, exposure and cumulative risk assessment. Science of the Total Environment 543, 19–27 (2016).
Howdeshell, K. L. et al. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol Sci 105, 153–65 (2008).
Christiansen, S. et al. Synergistic Disruption of External Male Sex Organ Development by a Mixture of Four Antiandrogens. Environ Health Perspect 117, 1839–1846 (2009).
Rider, C. V., Furr, J., Wilson, V. S. & Gray, L. E. A mixture of seven antiandrogens induces reproductive malformations in rats. Int J Androl 31, 249–262 (2008).
Rider, C. V. et al. Cumulative Effects of In Utero Administration of Mixtures of “Antiandrogens” on Male Rat Reproductive Development. Toxicologic Pathology 37, 100–113 (2009).
Rider, C. V., Furr, J. R., Wilson, V. S. & Gray, L. E. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int J Androl 33, 443–462 (2010).
Hannas, B. R. et al. Dose-Response Assessment of Fetal Testosterone Production and Gene Expression Levels in Rat Testes Following In Utero Exposure to Diethylhexyl Phthalate, Diisobutyl Phthalate, Diisoheptyl Phthalate, and Diisononyl Phthalate. Toxicological Sciences 123, 206–216 (2011).
Chen, C. Y. et al. Phthalates may promote female puberty by increasing kisspeptin activity. Hum Reprod 28, 2765–73 (2013).
Chang, W. H., Li, S. S., Wu, M. H., Pan, H. A. & Lee, C. C. Phthalates might interfere with testicular function by reducing testosterone and insulin-like factor 3 levels. Hum Reprod 30, 2658–70 (2015).
Hou, J. W. et al. The effects of phthalate and nonylphenol exposure on body size and secondary sexual characteristics during puberty. Int J Hyg Environ Health 218, 603–15 (2015).
Tsai, Y. A. et al. Effects of high di(2-ethylhexyl) phthalate (DEHP) exposure due to tainted food intake on pre-pubertal growth characteristics in a Taiwanese population. Environ Res 149, 197–205 (2016).
Kawasaki, T. Prediction of 24-hour urinary creatinine excretion from age, body weight and height of an individual and its application (vol 38, pg 567, 1991). Hypertension Research 34, 1067–1067 (2011).
Aylward, L. L., Kirman, C. R., Adgate, J. L., McKenzie, L. M. & Hays, S. M. Interpreting variability in population biomonitoring data: role of elimination kinetics. J Expo Sci Environ Epidemiol 22, 398–408 (2012).
Christensen, K. L. Y., Lorber, M., Koch, H. M., Kolossa-Gehring, M. & Morgan, M. K. Population variability of phthalate metabolites and bisphenol A concentrations in spot urine samples versus 24-or 48-h collections. Journal of Exposure Science and Environmental Epidemiology 22, 632–640 (2012).
Frederiksen, H. et al. Temporal Variability in Urinary Phthalate Metabolite Excretion Based on Spot, Morning, and 24-h Urine Samples: Considerations for Epidemiological Studies. Environ Sci Technol 47, 958–967 (2013).
Guo, Y. et al. Occurrence and Profiles of Phthalates in Foodstuffs from China and Their Implications for Human Exposure. J Agric Food Chem 60, 6913–6919 (2012).
Guo, Y., Wang, L. & Kannan, K. Phthalates and Parabens in Personal Care Products From China: Concentrations and Human Exposure. Arch Environ Contam Toxicol 66, 113–119 (2014).
Guo, Y. & Kannan, K. Comparative Assessment of Human Exposure to Phthalate Esters from House Dust in China and the United States. Environ Sci Technol 45, 3788–3794 (2011).
Chang, J. W. et al. Cumulative risk assessment for plasticizer-contaminated food using the hazard index approach (vol 189, pg 77, 2014). Environmental Pollution 196, 358–358 (2015).
Bao, J. Q. et al. Phthalate Concentrations in Personal Care Products and the Cumulative Exposure to Female Adults and Infants in Shanghai. Journal of Toxicology and Environmental Health-Part a-Current Issues 78, 325–341 (2015).
Janjua, N. R., Frederiksen, H., Skakkebaek, N. E., Wulf, H. C. & Andersson, A. M. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int J Androl 31, 118–30 (2008).
Parlett, L. E., Calafat, A. M. & Swan, S. H. Women’s exposure to phthalates in relation to use of personal care products. Journal of Exposure Science and Environmental Epidemiology 23, 197–206 (2013).
Philippat, C., Bennett, D., Calafat, A. M. & Picciotto, I. H. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environ Res 140, 369–376 (2015).
Huang, P. C. et al. Age and Gender Differences in Urinary Levels of Eleven Phthalate Metabolites in General Taiwanese Population after a DEHP Episode. PLoS One 10, e0133782 (2015).
Koch, H. M., Wittassek, M., Bruning, T., Angerer, J. & Heudorf, U. Exposure to phthalates in 5–6 years old primary school starters in Germany–a human biomonitoring study and a cumulative risk assessment. Int J Hyg Environ Health 214, 188–95 (2011).
Kranich, S. K., Frederiksen, H., Andersson, A. M. & Jorgensen, N. Estimated daily intake and hazard quotients and indices of phthtalate diesters for young danish men. Environ Sci Technol 48, 706–12 (2014).
Koch, H. M., Rossbach, B., Drexler, H. & Angerer, J. Internal exposure of the general population to DEHP and other phthalates–determination of secondary and primary phthalate monoester metabolites in urine. Environ Res 93, 177–85 (2003).
Koch, H. M., Gonzalez-Reche, L. M. & Angerer, J. On-line clean-up by multidimensional liquid chromatography-electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 784, 169–82 (2003).
Koch, H. M. et al. Di-n-butylphthalate and butylbenzylphthalate - urinary metabolite levels and estimated daily intakes: pilot study for the German Environmental Survey on children. Journal of Exposure Science and Environmental Epidemiology 17, 378–387 (2007).
Koch, H. M., Bolt, H. M., Preuss, R. & Angerer, J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol 79, 367–376 (2005).
U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS), Butylbenzyl phthalate (CASRN 85-68-7). http://www.epa.gov/iris/subst/0293.htm (1993a).
U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS), Di (2-ethylhexyl) phthalate (DEHP) (CASRN 117-81-7). http://www.epa.gov/iris/subst/0014.htm (1993b).
Benson, R. Hazard to the developing male reproductive system from cumulative exposure to phthalate esters--dibutyl phthalate, diisobutyl phthalate, butylbenzyl phthalate, diethylhexyl phthalate, dipentyl phthalate, and diisononyl phthalate. Regul Toxicol Pharmacol 53, 90–101 (2009).
Gray, L. E. Jr. et al. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci 58, 350–65 (2000).
Acknowledgements
We thank to our research assistants, Ms. Wan-Ting Chang, Ms. Wei-Yen Liang and Ms. Sih-Syuan Li for their assistance in data, specimen collection and sample pretreatment, and Mr. Chien-Jen Wang for his assistance in LC/MS-MS analysis. We also deeply grateful to the research collaboration of the NAHSIT team, Mr. Zheng Chen and others, and support of sampling by Health Promotion Administration, Ministry of Health and Welfare, Taiwan. We also thank for and funding support by the National Health Research Institutes (Grant No.: EH-102-PP-05, EH-103-PP-05, EH-104-PP-05, EM-105-PP-15).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: H.C.C., W.H.P., P.C.H. Performed the risk assessment models: J.W.C., P.C.H. Analyzed the data: J.W.C., C.C.L., P.C.H. Contributed materials/analysis tools: J.W.C., W.C.C., H.B.H., P.C.H. Wrote the paper: J.W.C., P.C.H. Contributed to critical revision of the manuscript: J.W.C., C.C.L., W.C.C., H.B.H., P.C.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Chang, JW., Lee, CC., Pan, WH. et al. Estimated Daily Intake and Cumulative Risk Assessment of Phthalates in the General Taiwanese after the 2011 DEHP Food Scandal. Sci Rep 7, 45009 (2017). https://doi.org/10.1038/srep45009
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep45009
- Springer Nature Limited
This article is cited by
-
Di(2-ethylhexyl) phthalate (DEHP) and thyroid: biological mechanisms of interference and possible clinical implications
Environmental Science and Pollution Research (2022)
-
Levels and temporal variations of urinary lead, cadmium, cobalt, and copper exposure in the general population of Taiwan
Environmental Science and Pollution Research (2019)
-
Urinary metabolomic profiling in rats exposed to dietary di(2-ethylhexyl) phthalate (DEHP) using ultra-performance liquid chromatography quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS)
Environmental Science and Pollution Research (2017)