Abstract
Recessive mutations in WD repeat domain 62 (WDR62) cause microcephaly and a wide spectrum of severe brain malformations. Disruption of the mouse ortholog results in microcephaly underlain by reduced proliferation of neocortical progenitors during late neurogenesis, abnormalities in asymmetric centrosome inheritance leading to neuronal migration delays, and altered neuronal differentiation. Spindle pole localization of WDR62 and mitotic progression are defective in patient-derived fibroblasts, which, similar to mouse neocortical progenitors, transiently arrest at prometaphase. Expression of WDR62 is closely correlated with components of the chromosome passenger complex (CPC), a key regulator of mitosis. Wild type WDR62, but not disease-associated mutant forms, interacts with the CPC core enzyme Aurora kinase B and staining of CPC components at centromeres is altered in patient-derived fibroblasts. Our findings demonstrate critical and diverse functions of WDR62 in neocortical development and provide insight into the mechanisms by which its disruption leads to a plethora of structural abnormalities.
Similar content being viewed by others
Introduction
Malformations of cerebral cortical development (MCD) are the outcome of disruptions in the precisely orchestrated series of events that occur during embryonic and early postnatal life and define the specification of neuronal identity and, ultimately, the execution of neuronal differentiation and connectivity both within and outside the cerebral cortex. MCD are neurodevelopmental disorders invariably causing developmental delay and were initially classified according to the phase at which disruptions of normal developmental events were thought to occur, impacting for example neural progenitor proliferation or apoptosis, neuronal migration, or cortical organization1,2. Progress in our fundamental understanding of normal cortical development3,4,5 along with advances in genetics, genomics, and imaging prompted a revision of the traditional classification6. Importantly, early genetic studies led to the realization that a subset of MCD disorders are indeed monogenic (e.g. refs 7, 8, 9, 10, 11), potentially offering unique insights into specific cellular functions involved in brain pathobiology. A wide spectrum of MCD are underlain by genetic lesions6, 17 of which are associated with autosomal recessive primary microcephaly (MCPH), a genetically heterogeneous disorder characterized by the clinical finding of a head circumference more than 2 standard deviations below the ethnically-matched age- and sex-related mean and thought to be secondary to abnormal neural progenitor proliferation or apoptosis6,12,13,14. MCPH2 (OMIM 604317), the second most common form of primary microcephaly, is caused by recessive mutations in WDR6215,16,17, a gene encoding a WD repeat-containing protein. Members of this large pan-eukaryotic protein family are thought to serve as rigid scaffolds coordinating multiprotein complex assemblies with diverse functions, including signal transduction, transcriptional regulation, cilia assembly, cell cycle control and apoptosis18,19,20,21. When mutations in WDR62 were initially discovered15, it became apparent that they cause a form of microcephaly invariably accompanied by a wide spectrum of additional and diverse cortical abnormalities, including pachygyria, thickened cortex, lissencephaly, and polymicrogyria, which were traditionally thought to be distinct, suggesting they can have a unified underlying genetic causation. More than 30 missense, nonsense, frameshift or splice site mutations mapping throughout the gene have been reported in patients around the world (refs 15, 16, 17,22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 and our unpublished findings). Like many other MCPH-associated proteins, WDR62 has been implicated in spindle maintenance, mitotic progression and maintenance of the neural progenitor pool33,34,35 and further shown to associate with c-Jun N-terminal kinase (JNK) and Aurora kinase A33,34,36,37,38,39. However, the mechanisms by which WDR62 dysfunction results in such diverse spectrum of structural brain abnormalities remain poorly understood.
We investigated WDR62 function using a mouse model and dermal fibroblasts from affected (homozygote) and unaffected (heterozygote) members of a family carrying a novel truncating mutation in WDR62. Our studies demonstrated that disruption of WDR62 impairs proliferation of neocortical progenitors during late neurogenesis underlain by abnormalities in asymmetric centrosome inheritance causing microcephaly in mice, and impairs mitotic cycle progression in patient-derived fibroblasts, which, similar to mouse neocortical progenitors, transiently arrest at prometaphase. Moreover, transcriptomic analyses identified genes with expression profiles significantly correlating with WDR62 across different human brain regions and developmental periods, including those encoding components of the Chromosome Passenger Complex (CPC), a major regulator of mitosis40. Functional analyses indicated transient interaction of WDR62 with AURKB, the enzymatic core of the CPC, which is significantly disrupted by MCPH-associated mutant forms of WDR62. Taken together, our findings provide novel mechanistic insight into WDR62 function in cortical development, demonstrating a complex effect on the cell cycle of neocortical progenitors, and may help explain the large spectrum of cortical malformations associated with WDR62 mutations causing microcephaly.
Results
Generation of mutant Wdr621–21 mice
Wdr62 mRNA is expressed in proliferating neural progenitors in the ventricular and subventricular zones (VZ and SVZ, in the latter at higher levels) of the dorsal telencephalon (pallium) as early as E11.5, in ventral (subpallial) progenitors that give rise to striatal neurons and cortical interneurons, and in proliferating granule cell precursors in the external granular layer (EGL) of the cerebellum at early postnatal stages (Supplementary Figure S1)15. In adult brain Wdr62 is highly restricted to the septum and medial thalamus (data not shown). In the developing human brain, WDR62 mRNA is enriched in the VZ, weakly expressed in the intermediate zone and cortical plate, whereas in adult human brain, it is detected throughout the neocortical layers (Supplementary Figure S1).
To investigate the function of WDR62 we generated a mouse carrying a homozygous mutation in Wdr62 from trapped ES cells (Wdr62Gt(CH0428)Wtsi) obtained from the Welcome Trust Sanger Institute (www.informatics.jax.org). The insertion site of the pGTOlxr gene-trap vector is between exons 21 and 22 of the Wdr62 locus (Supplementary Figure S1). Mice homozygous for the trapped allele express a truncated Wdr62 transcript as suggested by in situ hybridization with a cRNA probe that maps to the 5′ end of Wdr62, but not full-length Wdr62 (Supplementary Figure S1). Mice carrying the trapped allele (henceforth Wdr621-21) express a fusion protein consisting of amino acids 1–870 (of 1524) of WDR62 fused to the β-geo reporter, detected by x-gal staining, in accordance to the endogenous pattern (Supplementary Figure S1). Wdr621-21homozygous mice are viable but infertile, exhibiting dramatically reduced pregnancy rates (only two litters were produced in a period of six months by seven breeding pairs with one of the breeders being homozygous) and offspring number, likely reflecting defects in the germline. Several MCPH2- associated mutations identified in patients also lead to C-terminal truncations of variable length (e.g. S956CfsX3816, G1280AfsX2115,16, V1314RfsX18/V1313GfsX1716,17,25, V1402GfsX1215 and L1414LfsX4117) and a novel allele reported herein (D955AfsX112; see below).
Disruption of Wdr62 causes microcephaly in mice
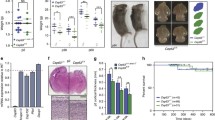
Wdr621-21/1-21mice were smaller than wild type at early postnatal stages, but no longer in adulthood (Supplementary Figure S2); however, their brain was smaller than normal from birth onwards (Fig. 1A,H and Supplementary Figure S2). They showed no spontaneous pathologies during a four-year period of observation. Measurements of cortical dimensions in the first postnatal week showed significant reduction in the length of the anterior to posterior (A-P) axis (wild type vs. Wdr621-21/1-21: P1: 7.5 mm ± 0 vs. 7 mm ± 0; P3: 5.75 mm ± 0.111 vs. 5 mm ± 0; p = 0.001, P6: 6.44 mm ± 0.055 vs. 6mm ± 0.083; p = 4.36 × 10−5) but no difference in the medial to lateral axis (M-L, measured at the widest part of the forebrain), with the exception of P3 brains (n = 3–9 pairs of wild type and Wdr621-21/1-21brains [P1: 6.83 mm ± 0.166 vs. 6.83 mm ± 0.166; P3: 7.33 mm ± 0.105 vs. 6.91 mm ± 0.083; p = 0.04, P6: 8.33 mm ± 0.083 vs. 8.22 mm ± 0.168; p = 0.512]) (Fig. 1B–D, and Supplementary Figure S2). In addition, whole brain and neocortical areas were significantly reduced (wild type vs. Wdr621-21/1-21: 32.888 mm2 ± 0.887 vs. 29.390 mm2 ± 0.991; p = 2.56 × 10−6 and 11.638 mm2 ± 0.637 vs. 10.376 mm2 ± 0.637; p = 1.608 × 10−4 respectively), measured at different levels along the rostral to caudal axis at P3 (n = 6 pairs, with six sections analyzed per brain) (Fig. 1E–G). In adulthood, the average brain weight (wild type vs. Wdr621-21/1-21: 0.476 gr ± 0.008 vs. 0.415 gr ± 0.02; p = 0.006, n = 5) or of cerebral cortical (0.176 gr ± 0.029 vs. 0.141 gr ± 0.035; p = 0.029, n = 3) weight was significantly reduced, as was the length of the A-P axis (8.375 mm ± 0.125 vs. 7.75 mm ± 0.144; p = 0.016, n = 4) (Fig. 1H–K). By applying the isotropic fractionator method41 we determined that total cell number in the neocortex and hippocampus (9 weeks of age, (n = 4 pairs of brains from littermates) was significantly reduced in Wdr621-21/1-21(15,731,250 ± 1,369,815) compared with wild type (18,559,375 ± 1,874,669, p = 0.01) (Fig. 1L). Nuclear volume was similar in neocortical neurons from wild type and Wdr621-21/1-21P3 brains immunostained with an antibody against BCL11B (aka CTIP2; a transcription factor enriched in subcerebrally projecting neurons in layers 5 and 6) (Supplementary Figure S2), suggesting that reduced cell number was the likely reason for smaller brain size in Wdr621-21/1-21mice. Cortical radial thickness was also reduced and neocortical cytoarchitecture was disrupted (Fig. 1M–T), with dense and compact upper cortical layers, especially at rostral levels, without, however, perturbed cell distribution (Supplementary Figure S2). These differences appeared to be transient but, although clearly evident and reproducible in the first postnatal week (Fig. 1O,P,S,T), were less pronounced in young adult brain (Fig. 1N,R). At late embryonic and early postnatal stages (P1, P3) the lateral ventricles of Wdr621-21/1-21brains appeared enlarged (Supplementary Figure S2). These observations demonstrated that WDR62 is a critical regulator of neocortical size in mice, as in humans, suggesting evolutionarily conserved function.
(A–L) Wdr621-21/1-21mice exhibit microcephaly. (A) Representative images of whole brains from WT and Wdr621-21/1-21 littermates at P3. (B) Brain weight is significantly decreased in Wdr621-21/1-21 pups compared with WT at P3 and P6. (C,D) Measurements of the anterior to posterior (A–P) white dotted brackets in (A) and medial to lateral (M-L, black dotted line in (A) axis length of WT and Wdr621-21/1-21 forebrains at P1, P3 and P6 show significant differences in (A–P) axis length (C) and a transient decrease in (M-L axis length at P3 (D) in Wdr621-21/1-21 forebrains compared with WT. Measurements were obtained from at least 3 and up to 9 pairs of brains per stage. (E–G) Total brain and cerebral cortical area are reduced in Wdr621-21/1-21mice. (E) Measurements were performed at six coronal levels along the A-P axis as indicated (n = 6 pairs, 6 sections per brain analyzed). (F,G) Significantly smaller brain (highlighted in F) and cerebral cortical (highlighted in G) areas in Wdr621-21/1-21 animals compared with WT. (H) Representative images of whole brains from WT and Wdr621-21/1-21 littermates at 9 weeks of age. (I–K) Reduced weight of whole brain (I) and cerebral cortex (J) and reduced length of A-P (K), but not M-L (see Figure S1), axis in Wdr621-21/1-21 mice compared with WT. (L) Decrease in total cell number in cerebral cortex and hippocampus of Wdr621-21/1-21 mice compared with WT as determined by the isotropic fractionator method (n = 4 pairs). (M–T) Wdr621-21/1-21mice exhibit abnormal cytoarchitecture in the cerebral cortex. Analyses of Nissl stained coronal brain sections at rostral (M–P) and caudal (Q–T) A-P levels at 5 weeks (M,N,Q,R), P7 (O,S) and P3 (P,T) reveals reduced thickness and abnormalities in neocortical cytoarchitecture in Wdr621-21/1-21brains compared with control (as indicated). At early postnatal stages, upper cortical layers appear condensed and deep layers are disorganized. These defects are more pronounced rostrally. In panels N–P and R–T, sections are shown in a medial (to the left) to lateral (to the right) orientation. Error bars represent s.e.m; ★p < 0.05, ★★p < 0.01, ★★★p < 0.005; NS: not significant (two-tailed Student’s t-test) Scale bar: (A,H): 1 mm; (M,Q): 0.5 mm; (P; applies to N–P and R–T): 0.1 mm.
WDR62 is required for proliferation of late-born cortical progenitors
A small brain with fewer cells may result from premature depletion of the neural progenitor pool and/or increased apoptosis. We first analyzed BrdU incorporation throughout embryogenesis, failing to detect significant differences in cortical progenitor cell proliferation in wild type vs. Wdr621-21/1-21 brain at E11.5, E13.5 and E15.5 following a 30-minute pulse (Fig. 2A,B). At E17.5, however, progenitor proliferation was reduced (Fig. 2C,D). TUNEL assay demonstrated more apoptotic cells in dorsal and ventral telencephalon of Wdr621-21/1-21 embryos compared with wild type at E15.5 (Fig. 2E,F), suggesting that the reduction in radial cortical thickness may be partially due to increased apoptosis in mid- and late neurogenesis. To assess cell cycle dynamics in dividing neural progenitors, we injected CldU at early (E13.5) and late (E16.5) stages of neurogenesis, and harvested the brains 24 hours later. We quantified the percentage of cells that had exited the cell cycle (CldU+Ki67−/CldU+) during the 24-hour chase period by double labeling with antibodies specific for CldU and Ki67, which labels all dividing cells. The 24-hour cell cycle exit index was not significantly different in embryos injected at E13.5 and harvested at E14.5 (Fig. 2G,H), but was reduced in Wdr621-21/1-21embryos injected at E16.5 and harvested at E17.5 compared with wild type (Fig. 2I,J). Additionally, the “leaving fraction” (BrdU+Ki67−)42, comprising cells in S phase at the time of BrdU injection that exited the cell cycle during the 30-minute chase period, was increased in Wdr621-21/1-21vs. wild type neocortex at E17.5 (Supplementary Figure S3). Quantification of mitotic cells (expressing phosphor-histone H3 [PH3]) at early (E13.5) and late (E17.5) stages of neurogenesis revealed that the mitotic index was increased in Wdr621-21/1-21embryos at E13.5, but decreased at E17.5 (Fig. 2K–N). The distribution of progenitors dividing in the ventricular surface (VS) or away from it was similar in the two genotypes at both stages examined (Fig. 2O). At the VS, we counted considerably more PH3+ cells in Wdr621-21/1-21embryonic brains at E13.5 compared with wild type (Fig. 2P). Therefore, WDR62 disruption appeared to impact early and late neural progenitors differently: at early stages, it resulted in increase in the number of progenitors undergoing mitosis without, however, significantly affecting proliferation or cell cycle exit, whereas by late neurogenesis, WDR62 disruption led to decreased proliferation and decreased 24-hour cell cycle exit index, yet increased “leaving fraction” over a 30-minute period, suggesting that some (but not all) late progenitors leave the cell cycle early. Taken together these observations suggest that WDR62 differentially impacts self-renewal and differentiation of early vs. late neocortical progenitors.
(A–D) BrdU incorporation analyses. (A,C) Coronal sections of wild type and Wdr621-21/1-21 embryonic brains at E13.5 (A) and E17.5 (C) immunostained with an antibody specific to BrdU. Embryonic neocortices were analyzed following a 30-minute BrdU pulse. (B,D) Quantification of the number of BrdU+ cells at E11.5, E13.5, E15.5 (B) and E17.5 (D) reveals decreased proliferation in Wdr621-21/1-21 brains compared with wild type at late stages of neocortical development (n = 3 pairs, 3–4 sections analyzed per brain). (E,F) Detection of apoptotic cells using the TUNEL assay. Quantification of cells stained with TUNEL in coronal brain sections (E) reveals increased apoptosis (F) at E15.5 in Wdr621-21/1-21 neocortex compared with wild type (n = 3 pairs of brains, 3 sections analyzed per brain; boxes in E delineate the area of each section where cells were counted). (G–J) Analyses of cell cycle exit. Immunofluorescence staining for CldU and Ki67 at E14.5 (G) and E17.5 (I) following a 24-hour CldU pulse. (H,J) Quantification of CldU+ Ki67−/CldU+ cells indicates reduced cell cycle exit at late stages of neurogenesis (E17.5) in Wdr621-21/1-21 compared with wild type brains (n = 3–4 pairs of brains, 3–4 sections analyzed per brain). (K–P) Analyses of progenitor cell division. (K,L) Immunofluorescence staining for PH3 at E13.5 (K) and E17.5 (L). (M–P) Quantification of PH3+ cells reveals significantly more mitotic cells in the developing neocortex of Wdr621-21/1-21 embryos compared with wild type littermates at E13.5 (M). In contrast, at the end of neurogenesis, the number of mitotic cells is decreased in Wdr621-21/1-21 neocortex (N). There are no significant differences in the distribution of PH3+ cells at the ventricular surface (VS) or at a distance from it between wild type and Wdr621-21/1-21 embryonic neocortex (O). Mitotic index (PH3+ cells/total number of cells) in the VS is increased in Wdr621-21/1-21 neocortex compared with wild type at E13.5, but not significantly different at E17.5 (P) (n = 3–4 pairs, 3–4 sections analyzed per brain). Number of DAPI+ cells counted per section in wild type vs. Wdr621-21/1-21: 1,075 vs. 934 (E13.5); 3,059 vs. 3,350 (E17.5). Error bars represent s.e.m; ★p < 0.05, ★★p < 0.01, ★★★p < 0.005; NS: not significant (two-tailed Student’s t-test) Scale bar: (A,C) 20 μm; (G,I,K,L) 30 μm.
WDR62 is required for proper centrosome inheritance
In non-neuronal cells, and dependent on cell line examined, WDR62 is detected at the spindle poles during at least some phases of mitosis15,17,22,27,33,35,43 and appears to associate with the centrosome, although it is not principally a centrosomal protein, at least in HeLa cells33. We re-examined WDR62 expression in neural progenitors, as we previously could not detect clear localization of WDR62 to the spindle poles in vivo using antibodies available at the time15. WDR62 can be detected at the spindle poles of cultured mouse neocortical progenitors, dependent on experimental conditions (Fig. 3A) and in human induced pluripotent stem cells (iPSCs) (Fig. 3B). This association with the spindle poles, together with the effects of WDR62 dysfunction on mitotic index and progenitor proliferation (Fig. 2), prompted us to investigate asymmetric centrosome inheritance as a possible mechanism underlying proliferation defects44. We utilized an in vivo assay (developed by S.-H. Shi44) to pulse-label centrosomes of proliferating neocortical progenitors with the photoconvertible fluorescent protein Kaede45 fused to the centriolar protein centrin 1 (Kaede-CETN1, gift from S-H Shi), in order to distinguish centrosomes containing the old mother centriole from those containing the new mother centriole (Fig. 3C). Kaede-CETN1 was electroporated at E15.5 and photoconverted at E16.5 (Fig. 3D,E and Supplementary Figure S4). In wild type as well as Wdr621-21/1-21neocortex at E18.5, more than 85% of labeled centrosomes contained both green and red (“yellow” upon merge) fluorescent centrioles; about 10% of centrosomes possessed only green fluorescent centrioles, whereas less than 5% were solely red fluorescent (corresponding to initially labeled progenitors that had not divided during this period), in agreement with previous observations44 (Fig. 3F). However, within the proliferating zones (VZ/SVZ; bin 1), the percentage of centrosomes with only green fluorescent centrioles (i.e. containing new mother centrioles only, having undergone two rounds of cell division following photoconversion) was higher in Wdr621-21/1-21compared with wild type (65% vs. 40%), whereas, the percentage of those retaining the old mother centriole (both green and red fluorescent, i.e. “yellow” upon merge) was lower (Fig. 3G–I). The opposite was true for the cortical plate (bin 3): compared with wild type, the Wdr621-21/1-21brains had fewer centrosomes containing green only centrioles (Fig. 3G–I) and a higher percentage of centrosomes containing both green and red centrioles (i.e. “yellow” upon merge) (Fig. 3G–I). These data suggested that, compared with wild type, in the proliferating zone of Wdr621-21/1-21neocortex more cells inherited the new mother centriole (green), which is typically inherited by the newly differentiating neurons migrating away from the VZ/SVZ. Accordingly, the distribution of cells containing new mother centrioles only (i.e. green fluorescent) within the developing neocortex (bin 1, 2 and 3), was different in Wdr621-21/1-21compared with wild type neocortex, with the former harboring significantly more in the proliferating zones and fewer in the migration zone and the cortical plate, suggesting abnormal migration, and possibly differentiation, of Wdr621-21/1-21neurons inheriting the new mother centriole.
(A,B) Immunofluorescence staining of murine neocortical progenitors (A) (prometaphase) and human induced pluripotent stem cells (iPSCs) (B) (metaphase) with antibodies specific to WDR62 and γ-tubulin. (C) Experimental procedure to visualize centrosomes with mother centrioles of different ages44. Centrosomes replicate once per cell cycle, giving rise to two centrosomes, each consisting of one mother and one daughter centriole transiently labeled via in utero electroporation with Kaede-CETN1 that is photoconvertable from green to red fluorescence. After the first replication, new centrosomes harbor one mother (red) and one daughter (green) centriole. After the second -and subsequent- cycles, centrosomes retaining the mother (red) and a newly synthesized (green) centriole appear yellow whereas those inheriting the daughter (green) and a newly synthesized centriole are green-fluorescent. (D,E) Representative images of the cortical wall of E18.5 WT (D) and Wdr621-21/1-21 (E) littermates electroporated with Keade-CETN1 at E15.5 and photoconverted at E16.5. Insets are digital magnification images of the areas delineated by dotted squares depicting centrosomes that are green-only, red-only, or yellow-fluorescent. Some cells show protein accumulation in the cytoplasm as well (See also Supplementary Figure 4). The cortical wall was divided into three equally sized bins (1: VZ/SVZ; 2: IZ; 3: cortical plate). (F,G) Quantification of the percentage of green-, yellow-, or red- (F) and of green- or yellow-fluorescent centrosomes, as a percentage of all centrosomes, within each bin (G). Wdr621-21/1-21 brains harbor significantly more green centrosomes in bin 1 and fewer in bin 3, and fewer yellow centrosomes in bin 1 and more in bin 3. (H,I) Distribution of green- and yellow centrosomes. Wdr621-21/1-21 brains harbor a higher percentage of yellow centrosomes in bin 3 (H). In bin 1, and in bins 2 and 3, they also harbor, respectively, a higher and a lower percentage of green centrosomes (I). (number of centromeres counted: 7,156 [WT] vs. 6,960 [Wdr621-21/1-21] from 6 sections per brain from three pairs of brains). Error bars represent s.e.m; ★p < 0.05, ★★p < 0.01, ★★★p < 0.005; NS: not significant (two-tailed Student’s t-test). Scale bar: (A) 12.5 μm; (B) 20 μm; (E) also applies to (D) 50 μm.
Abnormalities in neuronal differentiation in Wdr621-21/1-21mice
Disruption in proliferation and cell cycle progression of progenitors has been linked to perturbations in neuronal cell fate46,47,48,49,50. At mid-to-late embryonic stages, mRNA expression of molecular markers of neural progenitors or subsets of post-mitotic neurons was grossly normal in Wdr621-21/1-21 neocortex (Supplementary Figure S5). At early postnatal stages, however, expression of Satb2, a marker of callosal projection neurons51,52 appeared increased in Wdr621-21/1-21neocortex compared with wild type (Fig. 4A). We quantified SATB2-expressing neurons after dividing the neocortical wall into 10 equally sized bins (Fig. 4B). Although their distribution (SATB2+ cells in each bin over all SATB2+ cells) was similar in wild type and Wdr621-21/1-21neocortex (Fig. 4C), more cells expressed SATB2 in the latter both in deep (bins 8 and 9) but also more superficial (bins 2–4) parts (Fig. 4D), leading to an overall increase in the percentage of SATB2+ neurons (Fig. 4E). In agreement with this, the corpus callosum appeared thicker in Wdr621-21/1-21brains compared with wild type (Supplementary Figure S6). Furthermore, more neurons expressed TBR1 (a marker for most pyramidal neurons in layers 1–3 and 653) and, conversely, fewer expressed CTIP2 (subcerebrally projecting neurons;54) in Wdr621-21/1-21neocortex compared with wild type (Fig. 4F–I). At P21, and in adult, several cortical layer markers appeared normal (Supplementary Figure S6 and data not shown), suggesting that despite abnormal cytoarchitecture in the neonatal period, cortical lamination was grossly normal in adult Wdr621-21/1-21 mice. This is in contrast to what is observed in patients with WDR62-associated MCPH, who invariably present with diverse malformations of cortical development (e.g. refs 15, 16, 17). Taken together, these data suggested that loss of WDR62 in neural progenitors affects the differentiation of a subset of cortical neurons.
(A–I) Neuronal differentiation defects in Wdr621-21/1-21 brains revealed by in situ hybridization and immunofluorescence staining with markers of post-mitotic cortical neurons. (A) Representative images of coronal brain sections from wild type and Wdr621-21/1-21 brains processed with in situ hybridization for Satb2 at the stages indicated. Wdr621-21/1-21 brains show an expansion of Satb2 mRNA expression domain compared with wild type. (B–E) Representative immunofluorescence images of coronal sections from wild type and Wdr621-21/1-21 brains at P3 stained with an antibody specific to SATB2 (B). Quantification of SATB2+ cells within each of 10 equally sized bins in relation to the total number of SATB2+ cells demonstrates normal distribution (C). Quantification of cortical neurons that are SATB2+, in relation to the total number of cortical cells (DAPI+) within each bin, demonstrates an increase in number of SATB2+ cells in upper (bins 2–4) and deep layers (bins 8 and 9, mostly layer 6) (D). The overall fraction of SATB2+ neurons is increased in Wdr621-21/1-21 brains compared with wild type (n = 4 pairs, average number of DAPI+ cells counted per section: 5,469 [wild type] vs. 5,320 [Wdr621-21/1-21]) (E). (F–I) Representative immunofluorescence images of coronal sections from wild type and Wdr621-21/1-21 brains at P3 stained with antibodies specific to TBR1 (F) or CTIP2 (H). Quantification of the fraction of TBR1+ or CTIP2+ neurons, in relation to the total number of cortical cells (DAPI+), shows that the percentage of TBR1+ cells is increased in Wdr621-21/1-21 brains compared with wild type (n = 3 pairs, average number of DAPI+ cells counted per section: 3,341 [wild type] and 3,207 [Wdr621-21/1-21]) (G), whereas the percentage of CTIP2+ cells is decreased (I) (n = 4 pairs, average number of DAPI+ cells counted per section: 3,518 [wild type] vs. 3,504 [Wdr621-21/1-21]). Error bars represent s.e.m; ★p < 0.05, ★p < 0.01, ★★★p < 0.005; NS: not significant (two-tailed Student’s t-test). Scale bar: (A) shown on each panel): 0.5 mm; (B,E,G) 50 μm.
Whole-exome sequencing identifies a novel recessive mutation in WDR62 in patients with microcephaly and structural brain abnormalities
We previously reported six independent MCPH-associated mutations in WDR6215 and many more have been described since by others. We identified a family (NG1406) with two affected siblings displaying global developmental delay (Fig. 5A and Supplementary data). The index case is a 13-year-old full-term male, the product of a consanguineous (second-cousin) marriage. He presented for medical attention at 20 months of age owning to global developmental delay and was found on clinical examination to have microcephaly and dysmorphic face. Neuroimaging studies identified an array of developmental abnormalities including diffuse pachygyria, thickened cortex and hypoplasia of the corpus callosum. 3D computed tomography showed metopic synostosis (Fig. 5B,C). His 6-year-old brother displayed similar findings (Supplementary data). Whole-exome sequencing identified a novel frameshift mutation in WDR62, D955AfsX112 (henceforth WDR62exon23), caused by a 4 bp deletion in exon 23, resulting in a premature stop codon at position 1067; the mutation was confirmed to be homozygous in both affected subjects, and heterozygous in both parents, using Sanger sequencing (Fig. 5D). It was not observed in more than 1,800 Turkish control chromosomes, the ESP (exome sequencing project) or 1,000 genome databases, and was detected twice in heterozygous state in the ExAC database with 1.649E-05 allele frequency. The WDR62exon23 mutation results in a truncated protein that lacks 569 C-terminal amino acids.
(A–D) Characterization of kindred NG1406 with WDR62-associated microcephaly. (A) Pedigree structure depicting a second-cousin consanguineous union. Arrow indicates the index case. (B,C) Coronal T1-weighted images demonstrate pachygyria (B) and 3D reconstruction of computed tomography scanned images show metopic synostosis (C) in the index case. (D) Chromatograms obtained via Sanger sequencing analysis of the two affected patients, their healthy sibling, and their parents, along with wild type control DNA (as indicated). Sanger sequencing confirmed exome sequencing findings of a 4-bp deletion in exon 23 of WDR62 resulting in a frameshift (D955AfsX112; designated WDR62exon23). The mutation was confirmed as being homozygous in the two affected siblings and heterozygous in their parents and was absent from the healthy sibling. (E–I) Analyses of fibroblasts from family NG1406. (E,F) Fibroblast cultures established from the heterozygous parents and the two affected siblings immunostained with antibodies specific to WDR62 and γ-tubulin demonstrate dynamic expression of WDR62 during the mitotic cycle. In heterozygous cells (E), WDR62 is detected at the spindle poles in prometaphase through telophase; in WDR62exon23homozygous cells (F), WDR62 remains diffusely distributed. (G–I) Distribution of mitotic cells in different phases of the mitotic cycle in synchronized cultures. Increased fraction of mitotic cells in prometaphase and anaphase, and decreased fraction of mitotic cells in cytokinesis are observed in WDR62exon23homozygous compared with heterozygous cultures (G). The fraction of cells undergoing mitosis is increased in homozygous cultures (H). Fewer cells proceed to metaphase following nocodazole-mediated arrest at prometaphase and subsequent release in normal culture conditions in WDR62exon23homozygous as compared with heterozygous cultures (I). (J,K) Quantification of the distribution of cells in each phase of the mitotic cycle along the ventricular surface (VS) of the developing neocortex (stained with DAPI; J) reveals more mitotic cells in prometaphase in Wdr621-21/1-21embryos compared with wild type (K). Arrows in (J) indicate mitotic cells along the VS, which are digitally magnified in the corresponding insets. Error bars represent s.e.m; ★p < 0.05, ★★p < 0.01, ★★★p < 0.005 (two-tailed Student’s t-test). Scale bar: (E,F) 5 μm; (J) 30 μm.
Mutations in WDR62 disrupt mitotic cycle progression
We characterized WDR62 expression in primary fibroblasts obtained from skin biopsies from the NG1406 family above carrying the WDR62exon23 allele. In cells from the heterozygous unaffected parents, WDR62 was diffusely distributed in prophase, progressively localizing to the spindle and spindle poles at prometaphase through late metaphase/anaphase, and dispersing again during telophase and cytokinesis (Fig. 5E). In contrast, in cells from the homozygous affected siblings, WDR62 did not localize to the spindle poles (Fig. 5F). Mitotic spindle formation appeared normal in fibroblasts of either genotype, as well as in iPSCs reprogrammed from them (Supplementary Figure S7); very rarely, extra centrosomes were observed (Supplementary Figure S8F; low frequency precluded statistical analyses). The integrity of the spindle poles in homozygous fibroblasts was further demonstrated by normal localization of ASPM, whose loss of function causes primary microcephaly (MCPH5)55,56, as well as of CDK5RAP2, a pericentriolar protein linked to MCPH357,58 (Supplementary Figure S8). Moreover, the vast majority of centrosomes formed normally in WDR62exon23heterozygous and homozygous fibroblasts, as evidenced by normal expression of CEP135 (Figure S8), which also causes MCPH when mutated59,60. Recently, WDR62 was reported to shuttle CEP63 to the centrosome43, however, in WDR62exon23homozygous fibroblasts, CEP63 localization was not disrupted (Supplementary Figure S8). Finally, we observed an increase in red-fluorescent centrosomes in homozygous as compared with heterozygous fibroblasts following transfection with Kaede-CETN1 and photoconversion (Supplementary Figure S8) suggesting that WDR62 disruption delays cell cycle progression.
In synchronized cultures, we observed a higher frequency of WDR62exon23 homozygous fibroblasts in prometaphase and anaphase (Fig. 5G). This increase was only partly counterbalanced by a decrease in cells in cytokinesis (Fig. 5G), such that the fraction of cells in mitosis was significantly higher in WDR62exo23 homozygous cultures (Fig. 5H). Thus, after an initial delay, WDR62exon23 homozygous cells were able to complete mitosis. We examined the capacity of WDR62exon23fibroblasts to proceed into the mitotic cycle following nocodazole-mediated arrest in prometaphase and subsequent release into normal culture media for one hour and observed a delay in homozygous compared with heterozygous cultures as reflected in lower frequency of metaphase cells in the former (Fig. 5I). In agreement with these findings, we observed significantly more mitotic progenitors at prometaphase in the VS of E17.5 Wdr621-21/1-21neocortex compared with wild type (Fig. 5J,K). Taken together, these observations suggested that WDR62 disruption is associated with abnormalities in mitotic cell cycle progression in patient fibroblasts as well as in embryonic murine neocortical progenitors.
To evaluate the functional consequences of additional WDR62 mutations at the cellular level, we used HeLa cells, which express WDR62 at higher levels throughout mitosis (as early as prophase and until after telophase) compared with other cells, including fibroblasts (Supplementary Figure S9). These analyses revealed a previously unappreciated dynamic expression during mitosis, with WDR62 initially diffusely distributed, then associating with the spindle, and gradually concentrating to the spindle poles at metaphase. Upon overexpression in HeLa cells, localization of wild type WDR62 (detected by virtue of a C-terminal V5 tag) paralleled that of the endogenous protein, however, four MCPH2-associated mutant forms of WDR62 (W224S; S956CfsX38; V1402GfsX1215,16 and D955AfsX112 [this study], also carrying a V5 tag), failed to localize to the spindle poles (Supplementary Figure S10). On very rare occasions, we observed mitotic cells in which a small (not quantifiable) fraction of WDR62W224S and WDR62V1402GfsX12 mutant proteins, otherwise diffusely distributed, localized to the poles (data not shown). These results suggested that the predominant consequence of MCPH2-associated mutations is the disruption of the dynamic shuttling and subcellular localization of WDR62 during mitosis. These observations further support the conclusion that the failure to detect WDR62 expression in the spindle poles of patient-derived WDR62exon23 homozygous fibroblasts indeed reflects defective shuttling of the mutant protein.
WDR62 interacts with the Chromosome Passenger Complex
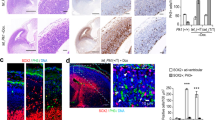
To gain further insight into the function of WDR62, we interrogated transcriptomic databases of developing and adult human brain61 for transcripts significantly correlating with WDR62 across developmental periods (15 total, ranging from embryonic to late adulthood) and different brain regions (transient prenatal structures and immature and mature forms of 16 brain regions, including 11 neocortical areas61) (Bonferoni corrected P < 0.01, n = 17,565). Functional annotation (DAVID Tools; http://david.abcc.ncifcrf.gov/62) suggested that positively correlated genes were enriched for those encoding cell cycle (Benjamini adjusted P = 7.06 × 10−44) and mitotic cell cycle (Benjamini adjusted P = 9.56 × 10−40) proteins. A group of 90 transcripts highly correlated (arbitrary correlation cutoff value 0.75) with WDR62 (top hit; correlation value 1) (Table 1) was enriched for core components of the chromosomal passenger complex (CPC), an evolutionarily conserved regulator of several processes required for mitosis40. In addition to the CPC core components Aurora kinase B (AURKB), CDCA8/borealin and BIRC5/survivin (respectively regulating CPC targeting and binding to the centromere), the group also includes bona fide AURKB/CPC substrates, that are either associated with the centromere and kinetochores (SKA1, ZWINT, NCD80, DIAPH3, and CENPE) (e.g. ref. 63), or encode kinesins (e.g. KIF2C, the mitotic centromere-associated kinesin; KIF20A, a mitotic kinesin required for CPC-mediated cytokinesis; and KIF4A, a chromokinesin that suppresses microtubule dynamics and is spatially and temporally regulated by AURKB64). These findings prompted us to investigate WDR62 in relation to CPC components using fibroblasts and HeLa cells. During mitosis and up to metaphase, we observed that a fraction of WDR62 transiently co-localizes with AURKB (which in addition to being enriched on chromosomes, it is also targeted to the spindle where it acts on microtubule-bound substrates65,66) as well as survivin (Fig. 6A and Supplementary Figure S11). Furthermore, a fraction of WDR62 could be observed at the kinetochores (Fig. 6I). The dynamic localization of AURKB and survivin67 was grossly similar in heterozygous and patient-derived WDR62exon23fibroblasts during mitosis (data not shown). Because depletion of microtubule-associated proteins is known to affect the level of CPC accumulation at centromeres68, we investigated AURKB and survivin staining in fibroblasts from the affected children relative to their unaffected parents. Homozygous fibroblasts at prophase exhibited a significantly decreased level of AURKB staining at centromeres relative to heterozygous cells (p = 0.0378), as well as an increased level of survivin staining at metaphase (p = 0.0056) (Fig. 6C–H). Staining was scored at each centromere relative to the level of CREST staining for centromeric proteins68. These findings suggested that upon WDR62 disruption, the levels of CPC components at centromeres were perturbed, prompting us to examine whether WDR62 might physically interact with AURKB. Immunoprecipitation of HeLa cell lysates transfected with wild type WDR62 along with AURKB or survivin showed interaction with AURKB (but not with survivin) (Fig. 6J and data not shown), which was disrupted upon expression of WDR62W224S and WDR62exon23, two MCPH2-associated mutant forms (Fig. 6K), suggesting that at least when overexpressed, the two proteins interact. Finally, we used Drosophila to examine possible genetic interactions of the fly orthologs of human WDR62 and AURKB, respectively Wdr62 (CG7337) and aurb. We employed the GAL4/UAS system, using Prospero-GAL4 to drive expression of Wdr62 and aurb RNAi (Wdr62-IR and aurb-IR) in neuroblasts (neural progenitor cells) and their newly born progeny (ganglion mother cells), which constitute the majority of cells in the developing larval brain69. Wdr62-IR resulted in markedly reduced brain size of 3rd instar larvae as compared with controls, confirming an evolutionarily conserved role for WDR62; in contrast, aurb-IR had no obvious effect on brain size. Combined Wdr62-IR and aurb-IR, however, resulted in formation of clusters of abnormally enlarged neuroblasts in 40% of brains analyzed (1–6 abnormal neuroblast clusters per brain, n = 10), identified by expression of the molecular marker Miranda; the more such clusters had developed in a brain, the smaller its size (Fig. 6L and Supplementary Figure S11). These observations suggested a genetic interaction of Wdr62 with aurb. When considered together, these observations provide converging evidence that WDR62 can interact with AURKB.
(A,B) Immunofluorescence staining of fibroblasts at prometaphase with antibodies specific to WDR62, AURKB, and survivin showing partial and transient co-localization of WDR62 with each protein. (C–H) The WDR62exon23 homozygous mutation affects levels of centromeric AURKB and survivin. (C,D) Representative images of heterozygous (top) and homozygous (bottom) fibroblasts stained with AURKB (C, prophase) or survivin (metaphase, D) (red; shown in grayscale) and FITC-conjugated CREST (green; shown in grayscale). (E,F) Schematic representation of method66 used to quantitate AURKB (E) and survivin (F) intensity. AURKB or survivin and CREST intensity was calculated by subtracting mean fluorescence intensity from a background (B) region (E,F) small circles) in the AURKB or survivin (red) and CREST (green) channel from the mean fluorescent intensity of total AURKB, survivin or CREST in the chromatin region (dotted outline on the merged images). The AURKB/CREST or survivin/CREST ratio was calculated according to the formula shown in the figure. (G,H) Box plot graphs of AURKB/CREST (G) and survivin/CREST (H) ratios in early prophase, prophase, prometaphase and metaphase. WDR62exon23 homozygous fibroblasts show significantly decreased levels of AURKB at prophase (G) and significantly increased levels of survivin at metaphase (H). Quantification of AURKB or survivin intensity using ImageJ was normalized to the CREST control (n = 20 to 35 cells per phase per genotype, *P < 0.05 by Mann-Whitney U test). (I) Immunofluorescence staining of fibroblasts shows partial and transient localization of WDR62 (red) in prometaphase kinetochores (CREST). (J,K) MCPH-associated mutant forms of WDR62 display reduced interaction with AURKB. Co-immunoprecipitation of WT (J) and mutant WDR62 (K) with AURKB. (L) Knockdown of WDR62 and AURKB orthologs in Drosophila. Expression of Wdr62-IR with prospero-Gal4 results in dramatically reduced brain size in 3rd instar larvae; aurb-IR has no effects on brain size. Combined Wdr62-IR and aurb-IR results in formation of clusters of abnormally enlarged neuroblasts, stained with Miranda (red); nuclei are stained with DAPI (blue). Images are 3D-projection of identical Z-sections. Scale bar (A,B) 5 μm; (C,D) 50 μm; (I) 10 μm; (L) 200 μm.
Discussion
In this study, we examine the effects of WDR62 disruption on neocortical development using a newly generated mouse model, identify a novel homozygous mutation in WDR62 in two affected siblings with microcephaly and severe brain malformations, and characterize its functional impact using patient-derived fibroblasts. We establish a critical role of WDR62 in proliferation of late neocortical progenitor cells and brain size and show that its disruption causes abnormalities in asymmetric centrosome inheritance leading to neuronal migration delays, and altered neuronal differentiation. We show that mitotic progression is defective in patient-derived fibroblasts, which, similar to mouse neocortical progenitors, transiently arrest at prometaphase. We also find that expression of WDR62 closely correlates to core components of the CPC, a major regulator of mitosis. We demonstrate that staining of CPC components at centromeres is altered in patient-derived fibroblasts and that, at least upon overexpression, mutant forms of WDR62 disrupt interactions with AURKB, the CPC enzymatic core. These findings highlight complex roles of WDR62 in mitotic cycle regulation, age-related progenitor maintenance, neuronal migration and differentiation, and may help explain the wide spectrum of structural brain abnormalities that uniquely characterize individuals with WDR62-associated microcephaly.
As in humans, disruption of WDR62 in mouse and Drosophila leads to microcephaly, highlighting its evolutionarily conserved function. Wdr621-21/1-21mice harbor smaller brains, with significantly shorter anterior-to-posterior neocortical axis length and fewer cells overall, reflecting decreased proliferation and decreased cell cycle exit of late progenitors and increased apoptosis. WDR62 dysfunction further impacts mitotic progression, as evidenced by enrichment of cells at prometaphase in murine mitotic progenitors in the VS and in dividing fibroblasts from individuals with MCPH2. In the murine neocortex, WDR62 loss disrupts asymmetric centrosome inheritance, a mechanism that has been previously linked to progenitor maintenance44. Disruption of centrosome inheritance also causes an imbalance in the behavior of newly postmitotic neurons, which, instead of migrating away, preferentially remain within the proliferating zones (Fig. 3). The underlying mechanism remains unknown, but the Drosophila Wdr62 ortholog also plays a role in maintaining centrosome asymmetry in neuroblasts and is required for microtubule stabilization and maintenance of microtubule organizing center activity on the apical interphase centrosome70. WDR62 disruption in patient-derived fibroblasts has no obvious impact on spindle or centrosome formation and proper localization of many pericentrosomal and centrosomal proteins; the localization of (endogenous or overexpressed) disease-associated mutant forms of WDR62 to the spindles poles, however, is impaired.
Previous studies also suggested that knockdown35 or genetic inactivation of WDR6234 leads to depletion of neural progenitors. Our findings in the Wdr621-21/1-21mouse partially confirm those from the Wdr621-14/1-14 mouse in which Wdr62 is disrupted after exon 1434; differences in insertion sites possibly account for the phenotypic discrepancies. Increased cell death and preferential loss of late neocortical progenitors occur in both models; however, we also uncovered a decrease in mitotic index and cell cycle exit as well as defects in neuronal differentiation manifested after birth, with several subtypes being differentially affected (Fig. 4). WDR62 disruption leads to delayed mitotic progression and prometaphase/metaphase arrest in Wdr621-14/1-14MEFs34 and we found that Wdr621-21/1-21 neocortical progenitors and patient-derived fibroblasts also transiently arrest in prometaphase. While Wdr621-14/1-14MEFs commonly display multipolar spindles, extra centrosomes and lagging chromosomes34, WDR62exon23homozygous fibroblasts harbor extra centrioles only very rarely (Supplementary Figure S8) and lack lagging chromosomes or multipolar spindles, suggesting that the domain encompassing WD repeats 11–13, encoded by exons 15 to 23, which is maintained in human, but not mouse fibroblasts, may be critical for spindle and centrosome assembly. It is known that prolonged prometaphase blocks daughter cell proliferation in human fibroblasts, despite normal completion of mitosis71 and mitotic delay correlates with preferential production of neurons at the expense of neural progenitors, as well as of apoptotic progeny in mouse neocortex50. Furthermore, disruption of normal cell cycle progression is known to cause apoptosis72 and abnormal cortical development46,47,48,50,73. Therefore, the documented and complex effects that WDR62 disruption has on the mitotic cycle of neural progenitors could explain the microcephaly with structural abnormalities in patients with MCPH2.
WDR62 disruption differentially impacts neural progenitors, with more pronounced effects in late neurogenesis. We did not examine whether all progenitors are equally affected at a given time, or whether the fraction of those affected eventually reaches a threshold. However, it is possible that even among progenitors at a given developmental stage, the effect of WDR62 is different, depending on their inherent proliferation properties. It is noteworthy that haploinsufficiency of the exon junction complex component Magoh only affects a subset of progenitors50, suggesting progenitor heterogeneity74,75. Pilaz et al.50 speculate that an internal clock capable of measuring mitosis duration may exist in neural progenitors; such clock may be differentially regulated during neurogenesis, especially in light of the observation that many genes whose expression highly correlates with WDR62 (Supplementary Table S1) display circadian expression pattern and have been implicated in transcriptional coordination of mitosis, albeit in a non-neural system76.
WD40 repeat-containing proteins coordinate multiprotein complex assemblies18,19. WDR62 interacts with JNK1/2 and Aurora A (AURKA) kinases, which, respectively, negatively regulate its association with microtubules, and control its localization to the spindle poles36,37,38,39. In vivo, WDR62 interacts genetically and physically with AURKA, and also associates with the mitotic spindle regulator TPX234,77. Furthermore, WDR62 and several other MCPH-associated proteins assemble at the centrosome where they promote centriole duplication43. We now demonstrate that WDR62 can interact with AURKB, the CPC core enzyme. It is known that depletion of any CPC component disrupts mitotic progression40,78,79,80, and WDR62 disruption causes a modest decrease in kinetochore levels of AURKB, and a significant increase in kinetochore levels of survivin in patient-derived fibroblasts. Although individual subunits of the CPC protein complex usually co-vary in this analysis68, it is possible that depletion of functional WDR62 has different effects on CPC subunits. The abnormalities in the AURKB and survivin relative ratios in WDR62exon23 homozygous fibroblasts suggest perturbed kinetochore function, especially as Wdr621-14/1-14MEFs were reported to exhibit increased tension across sister kinetochores34. In this regard, it is noteworthy that the kinetochore-associated genes CASC581 and CENPE82 have been associated with MCPH and that disruption in kinetochore localization is associated with microlissencephaly83 and that lissencephaly is a common comorbidity in MCPH2. Lastly, AURKB has been linked to embryonic stem cell pluripotency through phosphorylation-mediated inhibition of the master transcription factor OCT484. Whether AURKB kinetochore levels are also perturbed in neural progenitors upon WDR62 disruption is not known, and if this could have an effect on mitotic phosphorylation of transcription factors ultimately affecting cell fate remains to be investigated in future studies.
Our findings demonstrate complex effects of MCPH2-associated mutant forms of WDR62 on mitotic cycle progression, control of asymmetric centrosome inheritance and its association with the CPC, highlighting critical roles for WDR62 in brain morphogenesis and disease pathogenesis.
Methods
WDR621-21/1-21mice
Wdr621-21/1-21 mice were generated from trapped ES cells (Wdr62Gt(CH0428)Wtsi) obtained from the Welcome Trust Sanger Institute (www.informatics.jax.org) and maintained in accordance with the National Institutes of Health guidelines and approval of the Yale University Institutional Animal Care and Use Committee.
Brain fixation
Embryonic brains (except E18.5) were extracted and immersed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PBS) for 24 hours and up to several days, and processed, respectively, for immunohistochemistry and in situ hybridization or Nissl staining. Brains of E18.5 embryos, neonatal and postnatal mice were extracted after transcardiac perfusion with cold PBS followed by 4% PFA.
Brain measurements and quantification of cortical cell number
Brains were harvested at the indicated stages and fixed as described above. Measurements (in mm) were obtained along the anterior-most to posterior part of the forebrain (A-P axis length) and at the widest part of the forebrain (M-L axis length), as indicated in Fig. 1, using a Jameson Caliper.
Brains of 9-week-old mice were harvested and post-fixed by immersion in 4% PFA for 6–8 weeks. The cerebral cortex and the hippocampus were dissected and total cell numbers were estimated using the isotropic fractionator as previously described41. Briefly, samples were mechanically dissociated in a solution containing 40 mM sodium citrate and 1% Triton-X, turned into an isotropic suspension of isolated nuclei, and kept homogenous by agitation. The total number of nuclei in suspension, and therefore, the total number of cells in the original tissue, was estimated by determining the density of nuclei in small aliquots stained with DAPI, under the microscope with a 40X objective, using a hemocytometer for quantification.
Histological analyses and X-gal histochemistry
Brains at the indicated stages were fixed, extracted, and sectioned with a sledge cryomicrotome (Leica Microsystems, Wetzlar, Germany). Nissl staining was performed on serial sections by standard procedures. Embryonic brain sections were stained for β-galactosidase activity in a solution containing 0.5 mM potassium ferricyanide, 0.5 mM potassium ferrocyanide, 20 mM magnesium chloride, 0.1% Triton X-100, and 0.37 mg/ml X-gal.
BrdU labeling
Pregnant females were injected intraperitoneally with a solution of 5-bromo-2′-deoxyuridine (BrdU; 10 mg/ml in saline) at 20 μg/g of body weight and/or with 5-chloro-2′-deoxyuridine (CldU; 10 mg/ml in saline) at 42.5 μg/g of body weight. Brains were harvested and processed for immunohistochemistry or immunofluorescence with antibodies specific to BrdU and/or CldU.
TUNEL assay
Brain sections (36 μm thickness) were obtained with a cryomicrotome (Leica), air-dried, post-fixed in 4% PFA for 15 min, washed with PBS, incubated with Proteinase K (1 μg/ml) in Proteinase K buffer (100 mM Tris-HCl, pH 8.0, 50 mM EDTA pH 8.0) at 37 °C for 30 min, washed with PBS and processed using the Millipore S7100 apoptosis detection kit (Fisher Scientific) following the manufacturer’s instructions.
In situ hybridization
Embryonic and postnatal brains were fixed, respectively, by immersion in or transcardiac perfusion with 4% PFA, cryoprotected in 30% sucrose (in 4% PFA) and sectioned with a cryomicrotome (Leica). Human tissue was obtained from several sources, including the Human Fetal Tissue Repository at the Albert Einstein College of Medicine (New York, NY), fixed by immersion in 4% PFA and sectioned. Sections were processed for in situ hybridization as described previously85. RNA probes complementary to mouse transcripts were obtained from colleagues [Pax686, Tbr287, Cux288,89), Er81 (a.k.a. Etv1, Mouse Genome Informatics90, Tle491, claudin 11 (gift from S. Tsukita, Kyoto University, Japan), Notch1, reelin, Scip (gifts from E. Grove, University of Chicago), Rorb (gift from C. Ragsdale, University of Chicago)], or generated using oligonucleotide pairs as indicated below (for Wdr62, see ref. 15). cRNAs were labeled with digoxigenin-11-UTP. Sections were analyzed using a Stemi stereomicroscope or an AxioImager (Zeiss, Oberkochen, Germany) fitted with an AxioCam MRc5 digital camera. Images were captured using AxioVision software (Zeiss) and assembled in Adobe Photoshop.
Oligonucleotides used to generate the in situ probes as shown in Table 2.
Immunofluorescence - brain sections
For immunofluorescence staining of sections at prenatal stages, brains were harvested and fixed as described above. The brains were cryoprotected by sequential immersion in 15% and 30% sucrose in PBS, flash frozen into embedding medium (Tissue-Tek, Sakura Finetek USA, Torrance, CA) and sectioned using a cryostat (Leica). Sections (12 μm) were air-dried, post-fixed in 4% PFA for 10 min and washed with PBS 3 times for 5 min each, blocked in a solution containing 5% normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA), 1% bovine serum albumin, 0.1% glycine, 0.1% L-lysine supplemented with 0.3% Triton X-100 for 1 hour and then incubated in the above solution with the appropriate primary antibody overnight at 4 °C. Sections were washed 3 times in PBS for 10 min each and incubated with secondary antibodies [Alexa-Fluor conjugated (Molecular Probes; 1:300 dilution)] in blocking solution without detergent for 2 hours at room temperature. For immunofluorescence staining at postnatal stages, brains were harvested following transcardiac perfusion as described above, post-fixed in 4% PFA overnight and cryoprotected in 30% sucrose in PBS for several hours. Sections (50 μm) were obtained on a cryomicrotome (Leica) and processed free-floating. After washing 2 times in PBS, 5 min each, sections were incubated in blocking solution (same as above) with 0.9% Triton X-100 for 1–2 hours at room temperature, followed by incubation with the appropriate primary antibodies in the same blocking solution for 72 hours at 4 °C. Sections were washed 3 times with PBS, 15 min each and incubated with the appropriate secondary antibodies in blocking solution without detergent for 2 hours at room temperature.
Immunofluorescence – cells
Human fibroblasts, iPSCs, murine embryonic cortical neurons and HeLa cells were fixed with cold 4% PFA for 15 min at room temperature, washed 3 times with PBS, incubated in blocking solution (same as above) for 1 hour and then in primary antibody in blocking solution for 1 hour at room temperature. After 3 washes with PBS, secondary antibodies were applied in blocking solution without detergent for 1 hour at room temperature. Samples were mounted with Vectashield containing DAPI (Vector Laboratories), and imaged with a Zeiss LSM or a Leica TCS SP2 laser scanning confocal microscopy system.
Antibodies
WDR62 (Bethyl Laboratories, #301–560, 1:500); BrdU (Becton Dickinson, #BD-347580, 1:100; abcam, #ab1893, 1:100), CldU (Accurate Scientific, OB T00 30 G, 1:100), Ki67 (Thermo Scientific, SP6, RM-9106-S0, 1:200), SATB2 (Genway, #GWB-9F2D2F, 1:200), CTIP2 (abcam, #ab18465, 1:250), TBR1 (Santa Cruz, #sc-48816, 1:250), WDR62 (Bethyl Lab, #A301–560A, 1:500), gamma-tubulin (Sigma, #T6557, 1:500), ASPM (Novus Biologicals, #NB100–2278, 1:500), AIM-1 (Aurora kinase B; #BD-611083, 1:300), CEP63 (ED Millipore, #06–1292, 1:500), CEP135 (abcam, #ab75005, 1:1000), PH3 (Cell Signaling, #9701 S, 1:300), CDK5RAP2 (Bethyl, #IHC-00063, 1:100), BIRC5/survivin (Santa Cruz, #sc-17779, 1:100), CREST (Human anti-Centromere Proteins; Antibodies Incorporated, #15–235-F, 1:100); myc (for immunoprecipitation: rabbit polyclonal abcam #9106; for Western blot: monoclonal 9E10, 1:1,000); V5 (monoclonal TCM5, eBioscience #14–6796; for Western blot: 1:1,000; for immunofluorescence 1:500); V5 (polyclonal Millipore #AB3792 for immunofluorescence 1:600).
In utero electroporation and photoconversion
All surgical procedures were performed using sterile conditions and in accordance with IACUC approved protocols. In utero electroporation was performed using standard procedures92 and the photoconversion was performed as previously described44. The Kaede-centrin1 plasmid was a gift from S.-H. Shi (Memorial Sloan Kettering Cancer Center, New York).
Primary cultures of embryonic cortical neurons
Cortical leaves from E15.5 embryos were dissected in cold Hanks’ Balanced Salt Solution (HBSS) supplemented with 0.5% D-glucose and 25 mM HEPES (HBSS+). Minced cortical tissue samples were dissociated using Papain-Protease-DNAse I (PDD) solution (190 μl Papain, 50 mg Dispase II and 5 mg DNAse I per 50 ml of HBSS+) and incubated at 37 °C for 30–40 min. Samples were subjected to centrifugation at 500 g for 4 min and pellets were resuspended in 1 ml of Neurobasal medium supplemented with 1 mM Sodium Pyruvate, 2 mM Glutamax, Pen/Strep and B-27 Supplement and filtered through a 40-micron mesh filter. Cells were plated on glass coverslips coated with laminin and poly-ornithine and cultured in Neurobasal medium as above.
Skin biopsy and fibroblast culture
A 4-mm skin punch biopsy was taken under local anesthesia from the umbilical area of NG1406-1, NG1406-2, and their parents (NG1406-3, 1406-4) using standard procedures93,94. Samples were stored in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, #11965-084) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Gibco, #10438-026), 1% (1x) L-glutamine (Gibco, #25030-081), 2% (1x) Penicillin-Streptomycin (Gibco, #15140-122) and processed immediately on arrival. Following wash with PBS, the samples were minced and small fragments were laid onto the surface of 100 mm2 Petri dishes, in square areas marked by perpendicular lines made with scalpel blades. The fragments were allowed to air dry so as the dermis side of specimens adhered to the culture dish. The fragments were then cultured in DMEM with 10% FBS, 1% (1x) L-glutamine, 1% (1x) Penicillin-Streptomycin at 37 °C. The fibroblasts typically grew within 7–9 days.
Cell culture and synchronization
Human fibroblasts and HeLa cells were maintained in DMEM supplemented with 10% fetal bovine serum. To synchronize the fibroblast cultures, cells were rinsed with PBS and grown in serum-free medium for 18 hours, then seeded in gelatin-coated chamber slides and released into the cell cycle for 36 hours by addition of serum prior to fixation with 4% PFA and analysis. For arrest in prometaphase, asynchronous fibroblast cultures were treated with 0.2 μg/ml nocodazole (Sigma) for 14 hours, then fixed for analysis. Primary fibroblasts synchronized in prometaphase were washed and released into growth medium for 1 hour, fixed, and analyzed for progression to metaphase.
Nucleofection of fibroblasts
Fibroblasts were harvested by centrifugation and processed for transfection using a Nucleofector 2b device (Lonza, Allendale, NJ) and a kit for Human Dermal Fibroblasts (NHDF; Lonza) according to the manufacturer’s instructions. Cells (5 × 105) were nucleofected with 3 micrograms of Kaede-CETN1 plasmid44, plated on gelatin-coated chamber slides and exposed to violet light 24 hours later in order to photoconvert the fluorescent Kaede-CETN1 fusion protein. 48 hours after photoconversion, the cells were fixed and imaged with confocal microscopy (Leica DM6000 CFS). Images were processed using ImageJ and centrosomes were counted using Adobe Photoshop.
Immunoprecipitation
HeLa cells were cultured in DMEM with 10% fetal calf serum and transiently transfected with V5-tagged WT or mutant forms of WDR62 along with AURKB-3xMyc using Lipofectamine (Life Technologies, NY). 36 hours after transfection cells were treated with 0.2 μg/ml nocodazole (Sigma) for 12 hours, then were lysed using a Triton-X100 based buffer containing protease and phosphatase inhibitor cocktails (Calbiochem, San Diego, CA). Clarified supernatants were used for immunoprecipitation using Dynabeads (Life Technologies) according to the supplier’s instructions. Immunoprecipitates were analyzed by Western analysis using standard protocols. In brief, lysates were separated on gradient SDS-PAGE gels (4–16%, BioRad) and transferred to nitrocellulose or PVDF membranes. After blocking with 5% milk, the membranes were incubated first with rabbit anti-tag antibodies and then with a secondary HRP-conjugated anti-rabbit IgG (Jackson Immunoresearch Labs). Signals were detected with chemiluminescence reagent (BioRad).
Quantitative Analysis
All data obtained were analyzed using two-tailed Student’s t-test or Mann-Whitney U test with a significance level of at least P < 0.05 (★p < 0.05, ★★p < 0.01, ★★★p < 0.005). Values designate mean; error bars represent s.e.m.
Layer distribution analysis
To quantify the distribution of neurons, anatomically matched sections were selected. The neocortex of P3 (SATB2 immunofluorescence) or E18.5 brains (Kaede-CETN1 expression) was subdivided radially from the pia surface to the upper edge of the white matter, into 10 or 3 equally sized bins, respectively. SATB2+ cells or labeled centrosomes were counted in each bin as indicated in the respective graphs.
Imaging
Visualization of stained brain sections or cells was performed using a Stemi stereomicroscope or an AxioImager (Zeiss) fitted with an AxioCam MRc5 digital camera, or using a TCS SP5 confocal microscope (Leica). Images were captured using the AxioVision (Zeiss) or the integrated LAC (Leica) software. Confocal images were assembled using LAS AF lite (Leica Application Suite, Leica) or Image J (developed by Wayne Rasband, National Institutes of Health; http://imagej.nih.gov/ij). All images were processed with Image J and/or Adobe Photoshop.
Cloning and mutagenesis
Cloning of all genes in expression vectors was performed using the Gateway system (Life Sciences). Gateway BP and LR reactions were performed using BP and LR clonase, respectively, according to the vendor’s instructions. PCR reactions to amplify genes and/or gene fragments were performed using the high-fidelity Accuprime Pfx DNA polymerase (Life Sciences) while all mutagenesis reactions were performed using the high-fidelity Phusion DNA polymerase (New England Biolabs). Gateway B1/B2 PCR reactions were performed using primers below, in order to amplify the cDNA of interest. To design primers, we used the QuickChange Primer Design website (Agilent Technologies, Inc). B1/B2 PCR products containing the cDNA of the desired gene were purified using the PCR purification kit (Qiagen) and were recombined using BP clonase into the shuttle plasmid pDONR-zeo (Life Sciences). This yielded so-called cDNA ENTRY clones. All genes were fully sequenced at this stage. When needed, site-directed PCR mutagenesis was performed on cDNAs ENTRY clones, then fully sequenced. Once the sequence of any ENTRY clone was confirmed, the respective plasmid DNA was recombined using LR clonase into a common, CMV promoter-driven expression vector, pCDNA-DEST40 (Life Sciences), which carries a V5-HIS tandem tag at the 3′ end of the Gateway cassette. All B2 primers depicted below have no STOP codon, allowing tagging of all genes with the V5-HIS tandem tags present in pCDNA-DEST40.
pCAN3Myc-WDR62 (encoding the CS5 transcript of WDR62) was a gift from A. Aronheim (The Rappaport Faculty of Medicine, Technion, Israel)38. This cDNA was modified to include an in-frame 194 bp fragment that is present in the brain-enriched transcript (consensus WDR62 sequence, GenBank). To clone this fragment, the missing sequence was amplified from a fetal brain cDNA library using the primers below and the PCR product was inserted in the WDR62 cDNA sequence via standard PCR mutagenesis using Phusion polymerase.
Four mutations described in patients with MCPH2 were introduced in the wild type WDR62 cDNA. The same mutagenesis protocol and the respective primers (see below) were used to introduce the W224S missense mutation. To generate the shorter, truncated versions of WDR62 mutations were introduced in exon 23 (Asp955AlafsX112), intron 23 (S956CfsX38), and exon 31 (V1402GfsX12), using Gateway with reverse (B2) primers targeting the respective regions. Since the GACA deletion in exon 23 causes a frameshift that adds 112 amino acids until the next “stop” codon, we used PCR insertional mutagenesis to introduce the 112-amino acid tail to the previously made exon 23 deletion construct. The above PCR product obtained with the W62xMF/MR primer pair was purified and used for mutagenesis to insert the 112-amino acid tail “in-frame” at the end of the construct previously generated with the B2nsEX23 W primer. In all constructs the STOP codons at the end of the ORF were deleted to allow 3′ tagging of the respective cloned genes with the V5-His tandem peptide tags present within the DEST40 plasmid.
A full-length cDNA encoding Aurora Kinase B was purchased from Addgene (plasmid 23682: pDONR223-AURKB). The gene was recombined via LR reactions into the pcDNADEST40 Gateway expression vector. In addition, the V5-His tag was removed and replaced with a 3x Myc tag.
All constructs generated were confirmed by Sanger sequencing.
Gateway oligonucleotides used to subclone the WDR62 full length gene:
B1-W62: GGGGACAAGTTTGTACAAAAAAGCAGGCTTCGCCACCATGGCGGCCGTAGGGTCCGGAGGCTATG
B2nsW62: GGGGACCACTTTGTACAAGAAAGCTGGGTCCCAGTGCCCCCGTGCCTTCCTCCGCAC
Oligonucleotides used to insert the 194 bp fragment into the original clone:
F: GTGCTGGAGAAGTGGATCAACCTGAAG
R: GTCGAAGGTCAGTGCCACTGTATCTGG
Gateway oligonucleotides used to subclone WDR62 truncated proteins:
B2nsEX31W: GGGGACCACTTTGTACAAGAAAGCTGGGTCCCAGGGCTCACTCCAGCGGGCAGGGCTGCCCTGTAACA
B2nsEX23W: GGGGACCACTTTGTACAAGAAAGCTGGGTCGCTGTCTGTCCCTGTGACTGTCACTTC
Oligonucleotides used to perform the W224S mutagenesis
W224SF: CACTGTTGGGAACCGCCATGTGAGGTTCTCGTTCTTGGAAGTCTCCACTGAGACAAAG
W224SR: CTTTGTCTCAGTGGAGACTTCCAAGAACGAGAACCTCACATGGCGGTTCCCAACAGTG
Oligonucleotides used to add the “tail” of 112 amino acids in the exon 23 construct
W62 × 22MF: TACTCTCTGGAGGCAGAAGTGACAGTCACAGAGACAGCCAGTATTGCAGGAAGGAGGTG
W62 × 22MRns: AACTTTGTACAAGAAAGCTGGGTCGTGTCTCAAAGTGGTGGCGGAGGAACTTCT
Human subjects
The study protocol was approved by the Yale Human Investigation Committee (HIC) (IRB #9406007680). Institutional review board approvals for genetic and MRI studies, along with written informed consent from all study subjects, were obtained by the referring physicians at the participating institutions. Human fetal brain specimens at 19 weeks of gestation were obtained from the Human Fetal Tissue Repository at the Albert Einstein College of Medicine (CCI number 1993–042). Postmortem human brain specimens were obtained from tissue collections at the Department of Neuroscience at Yale School of Medicine.
Clinical histories of patients (NG1406 family)
The index case (NG1406-1) is a 13-year-old full term male who is the product of a second-cousin marriage. He presented for medical attention at 20 months of age owning to global developmental delay and was found on clinical examination to have microcephaly and dysmorphic facies. Neuroimaging studies identified an array of developmental abnormalities including diffuse pachygyria, thickened cortex and hypoplasia of the corpus callosum and three-dimensional computed tomography showed metopic synostosis. At 8 years of age, he appeared microcephalic (head circumference: 44.5 cm, <2nd centile; height: 116 cm, 3rd–10th centile; weight: 20 kg; 3rd–10th centile). He never developed the ability to speak. Large auricles were noted. The index case’s 6-year-old brother (NG1406-2) displayed similar symptoms. He was brought to medical attention as soon as he was born with microcephaly (head circumference: 31 cm, <2nd centile). He developed head control at 3 months and ability to sit without support at 7 months of age. Global developmental delay was detected with the DENVER developmental screening test, performed at 18 months. At age 2 years, head circumference was 41 cm (<2nd centile), height was 83 cm (10th–25th centile) and weight was 10 kg (<3rd centile). General examination was remarkable for global developmental delay, no ability to walk, hypoplastic forehead and large auricles. At age 3 1/2, head circumference was 46.5 cm (<2nd centile), height was 129 cm (10th–25th centile) and weight was 22 kg (<3rd centile). Like his brother, he exhibited microcephaly and no ability to speak fluently. Their routine blood studies, including neurometabolic assays, complete metabolic panel, complete blood count, and thyroid function tests were all within normal limits. Whole exome sequencing of the younger affected brother identified a novel homozygous frameshift mutation, D955AfsX112, caused by a 4 bp deletion in exon 23 of WDR62, resulting in a premature stop codon at position 1067; the mutation was confirmed to be homozygous in both affected subjects, and heterozygous in both parents, using Sanger sequencing. It was not observed in more than 1,800 Turkish control chromosomes, or in the ESP (exome sequencing project) and 1,000 genome databases.
Whole-exome capture and sequencing
Exome capture for the index case was performed using the NimbleGen 2.1 M human exome array (Roche Nimblegen, Inc., Madison, WI, USA) according to the manufacturer’s protocol along with modifications previously described15,95. Exome library sequencing was performed using the HiSeq2000 with barcoding technology, paired end analysis and six samples per lane. Image analysis and subsequent base calling were performed using the Illumina pipeline (version 1.8, Illumina, Inc.).
Exome data analysis
Analysis of the sequencing data was performed according to the previously described bioinformatics pipeline devised by our research team96.
Sanger sequencing
The mutation was evaluated by Sanger sequencing using standard protocols. Amplicons were cycle sequenced on ABI 9800 Fast Thermo cyclers (Applied Biosystems, Foster City, CA, USA), and post cycle sequencing clean-up was carried out with the CleanSEQ System (Beckman Coulter Genomics, Danvers, MA, USA). The amplicons were analyzed on a 3730 Å~L DNA Analyzer (Applied Biosystems Inc.).
Additional Information
How to cite this article: Sgourdou, P. et al. Disruptions in asymmetric centrosome inheritance and WDR62-Aurora kinase B interactions in primary microcephaly. Sci. Rep. 7, 43708; doi: 10.1038/srep43708 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Barkovich, A. J., Kuzniecky, R. I., Jackson, G. D., Guerrini, R. & Dobyns, W. B. Classification system for malformations of cortical development: update 2001. Neurology 57, 2168–2178 (2001).
Barkovich, A. J., Kuzniecky, R. I., Jackson, G. D., Guerrini, R. & Dobyns, W. B. A developmental and genetic classification for malformations of cortical development. Neurology 65, 1873–1887, doi: 01.wnl.0000183747.05269.2d [pii] 10.1212/01.wnl.0000183747.05269.2d (2005).
Sun, T. & Hevner, R. F. Growth and folding of the mammalian cerebral cortex: from molecules to malformations. Nat Rev Neurosci 15, 217–232, doi: 10.1038/nrn3707 (2014).
Hu, W. F., Chahrour, M. H. & Walsh, C. A. The diverse genetic landscape of neurodevelopmental disorders. Annu Rev Genomics Hum Genet 15, 195–213, doi: 10.1146/annurev-genom-090413-025600 (2014).
Silbereis, J. C., Pochareddy, S., Zhu, Y., Li, M. & Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 89, 248–268, doi: 10.1016/j.neuron.2015.12.008 (2016).
Barkovich, A. J., Guerrini, R., Kuzniecky, R. I., Jackson, G. D. & Dobyns, W. B. A developmental and genetic classification for malformations of cortical development: update 2012. Brain: a journal of neurology 135, 1348–1369, doi: 10.1093/brain/aws019 (2012).
Brunelli, S. et al. Germline mutations in the homeobox gene EMX2 in patients with severe schizencephaly. Nat Genet 12, 94–96, doi: 10.1038/ng0196-94 (1996).
des Portes, V. et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 92, 51–61 (1998).
Fox, J. W. et al. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron 21, 1315–1325 (1998).
Gleeson, J. G. et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell 92, 63–72 (1998).
Reiner, O. et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature 364, 717–721, doi: 10.1038/364717a0 (1993).
Gilmore, E. C. & Walsh, C. A. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol 2, 461–478, doi: 10.1002/wdev.89 (2013).
Alcantara, D. & O’Driscoll, M. Congenital microcephaly. Am J Med Genet C Semin Med Genet 166C, 124–139, doi: 10.1002/ajmg.c.31397 (2014).
Faheem, M. et al. Molecular genetics of human primary microcephaly: an overview. BMC medical genomics 8, 532, doi: 10.1186/1755-8794-8-S1-S4 (2015).
Bilguvar, K. et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature 467, 207–210, doi: nature09327 [pii] 10.1038/nature09327 (2010).
Yu, T. W. et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet 42, 1015–1020, doi: 10.1038/ng.683 (2010).
Nicholas, A. K. et al. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet 42, 1010–1014, doi: 10.1038/ng.682 (2010).
Neer, E. J., Schmidt, C. J., Nambudripad, R. & Smith, T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature 371, 297–300, doi: 10.1038/371297a0 (1994).
Smith, T. F., Gaitatzes, C., Saxena, K. & Neer, E. J. The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 24, 181–185, doi: S0968-0004(99)01384-5 [pii] (1999).
Li, D. & Roberts, R. WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases. Cell Mol Life Sci 58, 2085–2097 (2001).
Smith, T. F. Diversity of WD-repeat proteins. Subcell Biochem 48, 20–30 (2008).
Bhat, V. et al. Mutations in WDR62, encoding a centrosomal and nuclear protein, in Indian primary microcephaly families with cortical malformations. Clin Genet 80, 532–540, doi: 10.1111/j.1399-0004.2011.01686.x (2011).
Murdock, D. R. et al. Whole-exome sequencing identifies compound heterozygous mutations in WDR62 in siblings with recurrent polymicrogyria. Am J Med Genet 155, 2071–2077, doi: 10.1002/ajmg.a.34165 (2011).
Najmabadi, H. et al. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature 478, 57–63, doi: 10.1038/nature10423 (2011).
Kousar, R. et al. Mutations in WDR62 gene in Pakistani families with autosomal recessive primary microcephaly. BMC neurology 11, 119, doi: 10.1186/1471-2377-11-119 (2011).
Bacino, C. A., Arriola, L. A., Wiszniewska, J. & Bonnen, P. E. WDR62 missense mutation in a consanguineous family with primary microcephaly. Am J Med Genet A 158A, 622–625, doi: 10.1002/ajmg.a.34417 (2012).
Farag, H. G. et al. Abnormal centrosome and spindle morphology in a patient with autosomal recessive primary microcephaly type 2 due to compound heterozygous WDR62 gene mutation. Orphanet journal of rare diseases 8, 178, doi: 10.1186/1750-1172-8-178 (2013).
Memon, M. M. et al. A novel WDR62 mutation causes primary microcephaly in a Pakistani family. Molecular biology reports 40, 591–595, doi: 10.1007/s11033-012-2097-7 (2013).
Poulton, C. J. et al. Severe presentation of WDR62 mutation: is there a role for modifying genetic factors? Am J Med Genet A 164A, 2161–2171, doi: 10.1002/ajmg.a.36611 (2014).
Rupp, V., Rauf, S., Naveed, I., Windpassinger, C. & Mir, A. A novel single base pair duplication in WDR62 causes primary microcephaly. BMC medical genetics 15, 107, doi: 10.1186/s12881-014-0107-4 (2014).
McDonell, L. M. et al. The utility of exome sequencing for genetic diagnosis in a familial microcephaly epilepsy syndrome. BMC neurology 14, 22, doi: 10.1186/1471-2377-14-22 (2014).
Bastaki, F. et al. Novel splice-site mutation in WDR62 revealed by whole-exome sequencing in a Sudanese family with primary microcephaly. Congenit Anom (Kyoto) 56, 135–137, doi: 10.1111/cga.12144 (2016).
Bogoyevitch, M. A. et al. WD40-repeat protein 62 is a JNK-phosphorylated spindle pole protein required for spindle maintenance and timely mitotic progression. Journal of cell science 125, 5096–5109, doi: 10.1242/jcs.107326 (2012).
Chen, J. F. et al. Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nature communications 5, 3885, doi: 10.1038/ncomms4885 (2014).
Xu, D., Zhang, F., Wang, Y., Sun, Y. & Xu, Z. Microcephaly-associated protein WDR62 regulates neurogenesis through JNK1 in the developing neocortex. Cell reports 6, 104–116, doi: 10.1016/j.celrep.2013.12.016 (2014).
Cohen-Katsenelson, K., Wasserman, T., Khateb, S., Whitmarsh, A. J. & Aronheim, A. Docking interactions of the JNK scaffold protein WDR62. Biochem J, doi: 10.1042/BJ20110284 (2011).
Lim, N. R. et al. Opposing roles for JNK and Aurora A in regulating WD40-Repeat Protein 62 association with spindle microtubules. Journal of cell science 128, 527–540, doi: 10.1242/jcs.157537 (2015).
Wasserman, T. et al. A novel c-Jun N-terminal kinase (JNK)-binding protein WDR62 is recruited to stress granules and mediates a nonclassical JNK activation. Mol Biol Cell 21, 117–130, doi: E09-06-0512 [pii] 10.1091/mbc.E09-06-0512 (2010).
Zhang, F. et al. A Novel c-Jun N-terminal Kinase (JNK) Signaling Complex Involved in Neuronal Migration during Brain Development. J Biol Chem 291, 11466–11475, doi: 10.1074/jbc.M116.716811 (2016).
Carmena, M., Wheelock, M., Funabiki, H. & Earnshaw, W. C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol 13, 789–803, doi: 10.1038/nrm3474 (2012).
Herculano-Houzel, S. & Lent, R. Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. The Journal of neuroscience: the official journal of the Society for Neuroscience 25, 2518–2521, doi: 10.1523/JNEUROSCI.4526-04.2005 (2005).
Hasan, S. M. et al. Mcl1 regulates the terminal mitosis of neural precursor cells in the mammalian brain through p27Kip1. Development 140, 3118–3127, doi: 10.1242/dev.090910 (2013).
Kodani, A. et al. Centriolar satellites assemble centrosomal microcephaly proteins to recruit CDK2 and promote centriole duplication. Elife 4, doi: 10.7554/eLife.07519 (2015).
Wang, X. et al. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461, 947–955, doi: 10.1038/nature08435 (2009).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc Natl Acad Sci USA 99, 12651–12656, doi: 10.1073/pnas.202320599 (2002).
Lange, C., Huttner, W. B. & Calegari, F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell 5, 320–331, doi: 10.1016/j.stem.2009.05.026 (2009).
Pilaz, L. J. et al. Forced G1-phase reduction alters mode of division, neuron number, and laminar phenotype in the cerebral cortex. Proc Natl Acad Sci USA 106, 21924–21929, doi: 10.1073/pnas.0909894106 (2009).
Arai, Y. et al. Neural stem and progenitor cells shorten S-phase on commitment to neuron production. Nature communications 2, 154, doi: 10.1038/ncomms1155 (2011).
Mao, H. et al. Rbm8a haploinsufficiency disrupts embryonic cortical development resulting in microcephaly. The Journal of neuroscience: the official journal of the Society for Neuroscience 35, 7003–7018, doi: 10.1523/JNEUROSCI.0018-15.2015 (2015).
Pilaz, L. J. et al. Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain. Neuron 89, 83–99, doi: 10.1016/j.neuron.2015.12.007 (2016).
Alcamo, E. A. et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57, 364–377, doi: 10.1016/j.neuron.2007.12.012 (2008).
Britanova, O. et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57, 378–392, doi: 10.1016/j.neuron.2007.12.028 (2008).
Hevner, R. F. et al. Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29, 353–366 (2001).
Arlotta, P. et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo . Neuron 45, 207–221, doi: 10.1016/j.neuron.2004.12.036 (2005).
Bond, J. et al. ASPM is a major determinant of cerebral cortical size. Nat Genet 32, 316–320, doi: 10.1038/ng995 (2002).
Kouprina, N. et al. The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum Mol Genet 14, 2155–2165, doi: 10.1093/hmg/ddi220 (2005).
Bond, J. et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet 37, 353–355, doi: 10.1038/ng1539 (2005).
Fong, K. W., Choi, Y. K., Rattner, J. B. & Qi, R. Z. CDK5RAP2 is a pericentriolar protein that functions in centrosomal attachment of the gamma-tubulin ring complex. Mol Biol Cell 19, 115–125, doi: 10.1091/mbc.E07-04-0371 (2008).
Hussain, M. S. et al. A Truncating Mutation of CEP135 Causes Primary Microcephaly and Disturbed Centrosomal Function. American journal of human genetics 90, 871–878, doi: 10.1016/j.ajhg.2012.03.016 (2012).
Lin, Y. C. et al. Human microcephaly protein CEP135 binds to hSAS-6 and CPAP, and is required for centriole assembly. The EMBO journal 32, 1141–1154, doi: 10.1038/emboj.2013.56 (2013).
Kang, H. J. et al. Spatio-temporal transcriptome of the human brain. Nature 478, 483–489, doi: 10.1038/nature10523 (2011).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57, doi: 10.1038/nprot.2008.211 (2009).
Cheng, L. et al. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Developmental cell 20, 342–352, doi: 10.1016/j.devcel.2011.01.008 (2011).
Nunes Bastos, R. et al. Aurora B suppresses microtubule dynamics and limits central spindle size by locally activating KIF4A. The Journal of cell biology 202, 605–621, doi: 10.1083/jcb.201301094 (2013).
Tseng, B. S., Tan, L., Kapoor, T. M. & Funabiki, H. Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Developmental cell 18, 903–912, doi: 10.1016/j.devcel.2010.05.018 (2010).
Iyer, J. & Tsai, M. Y. A novel role for TPX2 as a scaffold and co-activator protein of the Chromosomal Passenger Complex. Cell Signal 24, 1677–1689, doi: 10.1016/j.cellsig.2012.04.014 (2012).
Carmena, M. & Earnshaw, W. C. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol 4, 842–854, doi: 10.1038/nrm1245 (2003).
Papini, D. et al. TD-60 links RalA GTPase function to the CPC in mitosis. Nature communications 6, 7678, doi: 10.1038/ncomms8678 (2015).
Isshiki, T., Pearson, B., Holbrook, S. & Doe, C. Q. Drosophila neuroblasts sequentially express transcription factors which specify the temporal identity of their neuronal progeny. Cell 106, 511–521 (2001).
Ramdas Nair, A. et al. The Microcephaly-Associated Protein Wdr62/CG7337 Is Required to Maintain Centrosome Asymmetry in Drosophila Neuroblasts. Cell reports 14, 1100–1113, doi: 10.1016/j.celrep.2015.12.097 (2016).
Uetake, Y. & Sluder, G. Prolonged prometaphase blocks daughter cell proliferation despite normal completion of mitosis. Curr Biol 20, 1666–1671, doi: 10.1016/j.cub.2010.08.018 (2010).
Insolera, R., Bazzi, H., Shao, W., Anderson, K. V. & Shi, S. H. Cortical neurogenesis in the absence of centrioles. Nat Neurosci 17, 1528–1535, doi: 10.1038/nn.3831 (2014).
Takahashi, T., Nowakowski, R. S. & Caviness, V. S. Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. The Journal of neuroscience: the official journal of the Society for Neuroscience 15, 6046–6057 (1995).
Franco, S. J. & Muller, U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron 77, 19–34, doi: 10.1016/j.neuron.2012.12.022 (2013).
Tyler, W. A., Medalla, M., Guillamon-Vivancos, T., Luebke, J. I. & Haydar, T. F. Neural precursor lineages specify distinct neocortical pyramidal neuron types. The Journal of neuroscience: the official journal of the Society for Neuroscience 35, 6142–6152, doi: 10.1523/JNEUROSCI.0335-15.2015 (2015).
Siffroi-Fernandez, S. et al. Functional genomics identify Birc5/survivin as a candidate gene involved in the chronotoxicity of cyclin-dependent kinase inhibitors. Cell Cycle 13, 984–991, doi: 10.4161/cc.27868 (2014).
Lim, N. R. et al. Aurora A phosphorylation of WD40-repeat protein 62 in mitotic spindle regulation. Cell Cycle 15, 413–424, doi: 10.1080/15384101.2015.1127472 (2016).
Adams, R. R., Maiato, H., Earnshaw, W. C. & Carmena, M. Essential roles of Drosophila inner centromere protein (INCENP) and aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. The Journal of cell biology 153, 865–880 (2001).
Gassmann, R. et al. Borealin: a novel chromosomal passenger required for stability of the bipolar mitotic spindle. The Journal of cell biology 166, 179–191, doi: 10.1083/jcb.200404001 (2004).
Vader, G., Kauw, J. J., Medema, R. H. & Lens, S. M. Survivin mediates targeting of the chromosomal passenger complex to the centromere and midbody. EMBO Rep 7, 85–92, doi: 10.1038/sj.embor.7400562 (2006).
Genin, A. et al. Kinetochore KMN network gene CASC5 mutated in primary microcephaly. Hum Mol Genet 21, 5306–5317, doi: 10.1093/hmg/dds386 (2012).
Mirzaa, G. M. et al. Mutations in CENPE define a novel kinetochore-centromeric mechanism for microcephalic primordial dwarfism. Hum Genet 133, 1023–1039, doi: 10.1007/s00439-014-1443-3 (2014).
Bakircioglu, M. et al. The essential role of centrosomal NDE1 in human cerebral cortex neurogenesis. American journal of human genetics 88, 523–535, doi: 10.1016/j.ajhg.2011.03.019 (2011).
Shin, J. et al. Aurkb/PP1-mediated resetting of Oct4 during the cell cycle determines the identity of embryonic stem cells. Elife 5, doi: 10.7554/eLife.10877 (2016).
Louvi, A., Nishimura, S. & Gunel, M. Ccm3, a gene associated with cerebral cavernous malformations, is required for neuronal migration. Development 141, 1404–1415, doi: 10.1242/dev.093526 (2014).
Gotz, M., Stoykova, A. & Gruss, P. Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, 1031–1044 (1998).
Englund, C. et al. Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. The Journal of neuroscience: the official journal of the Society for Neuroscience 26, 9184–9195 (2006).
Nieto, M. et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol 479, 168–180, doi: 10.1002/cne.20322 (2004).
Zimmer, C., Tiveron, M. C., Bodmer, R. & Cremer, H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex 14, 1408–1420, doi: 10.1093/cercor/bhh102 (2004).
Weimann, J. M. et al. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron 24, 819–831 (1999).
Yao, J. et al. Combinatorial expression patterns of individual TLE proteins during cell determination and differentiation suggest non-redundant functions for mammalian homologs of Drosophila Groucho. Development, growth & differentiation 40, 133–146 (1998).
Tabata, H. & Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience 103, 865–872 (2001).
Levitt, J., Bernardo, S. & Whang, T. Videos in clinical medicine. How to perform a punch biopsy of the skin. N Engl J Med 369, e13, doi: 10.1056/NEJMvcm1105849 (2013).
Zuber, T. J. Punch biopsy of the skin. Am Fam Physician 65, 1155–1158, 1161–1152, 1164 (2002).
Choi, M. et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci USA 106, 19096–19101, doi: 10.1073/pnas.0910672106 (2009).
Clark, V. E. et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339, 1077–1080, doi: 10.1126/science.1233009 (2013).
Acknowledgements
We thank the family for participating in this study. We thank K. Bilguvar for discussions, A. Aronheim (Rappaport Faculty of Medicine, Technion, Israel) and S.-H. Shi (Memorial Sloan Kettering Cancer Center, New York, NY) for plasmids, S. Herculano-Houzel (University of Rio de Janeiro, Brazil) for advice on the Isotropic Fractionator method, and C. Chabu for help with confocal microscopy. This work used the Yale Center for Genome Analysis, supported by NIH Grant U54HG006504 (Yale Center for Mendelian Disorders, to R. P. Lifton, M.G., M. Gerstein and S. S. Mane). This work was supported by NIH grant R01HD075822 (to A.L.).
Author information
Authors and Affiliations
Contributions
A.L. conceived and designed the study and P.S., K.M.-G. and A.L. designed the experiments. P.S., K.M.-G., I.S., C.C., K.G., K.I., A.O.C. and A.L. performed the experiments. O.H. and J.L.Q. generated reagents. B.T. identified, consented and recruited the study subjects and provided clinical data. N.S. contributed transcriptome data. P.S., K.M.-G. and A.L. analyzed the data and A.O.C and M.G. analyzed the genetics data. P.S. and A.L. wrote the paper and P.S., N.S., A.O.C., M.G. and A.L. revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Sgourdou, P., Mishra-Gorur, K., Saotome, I. et al. Disruptions in asymmetric centrosome inheritance and WDR62-Aurora kinase B interactions in primary microcephaly. Sci Rep 7, 43708 (2017). https://doi.org/10.1038/srep43708
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep43708
- Springer Nature Limited