Abstract
The ‘thermal grill illusion’ (TGI) is a unique cutaneous sensation of unpleasantness, induced through the application of interlacing warm and cool stimuli. While previous studies have investigated optimal parameters and subject characteristics to evoke the illusion, our aim was to examine the modulating effect as a conditioning stimulus. A total of 28 healthy control individuals underwent three testing sessions on separate days. Briefly, 15 contact heat stimuli were delivered to the right hand dorsum, while the left palmar side of the hand was being conditioned with either neutral (32 °C), cool (20 °C), warm (40 °C), or TGI (20/40 °C). Rating of perception (numeric rating scale: 0–10) and evoked potentials (i.e., N1 and N2P2 potentials) to noxious contact heat stimuli were assessed. While cool and warm conditioning decreased cortical responses to noxious heat, TGI conditioning increased evoked potential amplitude (N1 and N2P2). In line with other modalities of unpleasant conditioning (e.g., sound, visual, and olfactory stimulation), cortical and possibly sub-cortical modulation may underlie the facilitation of contact heat evoked potentials.
Similar content being viewed by others
Introduction
In 1896, Torsten Thunberg first described the paradoxical sensation of heat in response to interlaced warm and cool stimulation. Since these seminal observations, the “thermal grill illusion” (TGI) has been described extensively using various modern stimulating devices. Of defining characteristics, the TGI is reported as a uniquely “unpleasant” somatosensory experience1,2,3,4,5,6,7,8. Central disinhibition related to persistent activation in polymodal nociceptive spinothalamic cells, coupled with reductions of activity in innocuous thermoreceptive spinothalamic cells has been proposed as underlying the unpleasantness of the TGI6,9,10. Neuroimaging investigations have demonstrated activation in prominent brain areas, including the anterior cingulate cortex (ACC)11. Activation in the ACC during the TGI is notable because this area has been implicated in processing unpleasant visual, auditory, and olfactory stimuli12,13,14,15.
Investigating the conditioning effects of afferent stimuli on responses to noxious input has generated a wealth of knowledge regarding endogenous pathways modulating pain. The notion of “anti-nociception” has been largely explored using conditioning somatosensory input, which is typically perceived as both painful and unpleasant16,17,18,19. In contrast, “pro-nociception” has been demonstrated using unpleasant auditory, visual, and olfactory conditioning (i.e., special senses)12,14,20,21,22,23,24. To our knowledge, no studies have bridged anti- and pro-nociception using a purely unpleasant somatosensory conditioning stimulus (i.e., not perceived as painful).
The objective of the current study was to address the heterotopic conditioning effect of an unpleasant somatosensory afferent stimulus (i.e., interlaced hot and cool bars) on responses to noxious contact heat stimulation. In line with other forms of unpleasant sensory conditioning, we hypothesized that the TGI would result in increased responses to noxious afferent stimulation. To explore changes in cortical activity, the conditioning effect of the TGI was examined using contact heat evoked potentials (CHEPs).
Material and Methods
Subjects
A total of 32 healthy control individuals (31.2 ± 6.9 years; gender: 13 female, 19 male) were recruited. Inclusion criteria were (1) age between 18–40 years, (2) being naïve to TGI and CHEPs, and (3) fluent in English or German. Exclusion criteria comprised pregnancy, intake of any medication (except birth control), and previous participation in a TGI or CHEPs study. All participants provided written informed consent and all procedures described below were in accordance with the Declaration of Helsinki and approved by the local ethics board ‘Kantonale Ethikkommission Zürich, KEK’ (ref. number: EK-04/2006; cinicaltrial.gov number: NCT02138344).
Study protocol
Prior to the commencement of the study, all individuals were interviewed to measure pain catastrophizing using the German or English version of the pain catastrophizing scale (PCS)25. The PCS investigates individuals catastrophic thinking related to painful experiences. The overall reliability and validity of the PCS has been demonstrated in earlier studies26,27,28,29. Previous studies have demonstrated significant correlations with endogenous pain modulation and PCS30,31,32, and the TGI and PCS33.
The study consisted of three different testing days differing only by the conditioning stimulation applied (Fig. 1). On the first day, all individuals were familiarized with the stimulation devices (CHEPS Stimulator and Thermal Grill device) and the temperatures (i.e., neutral, warm, cool, and interlaced cool/warm) that would be used for conditioning. All stimulation conditions were delivered using an identical device. Bars were set to either 32 °C (neutral), 20 °C (cool), 40 °C (warm), or alternating 20/40 °C (i.e., the TGI). Each condition was presented to the individuals for 30 s followed by a short interview to assess whether the conditions were perceived as unpleasant or painful (yes/no). If, and only if, a subject indicated unpleasantness or pain, they were then asked to rate the pain/unpleasantness by means of a numeric rating scale ranging from 0 (not unpleasant/painful at all) to 10 (very unpleasant or most unbearable pain). We believe that this is an important distinction from studies that cue subjects to rate unpleasantness/pain without first identifying if the sensation was unpleasant/painful34. Independent of their reported perception, all individuals were asked to describe the various modalities from a list of descriptors (e.g., warm, burning, cool, freezing, sharp, itching, and unpleasant). The goal of the familiarization phase was to reduce the novelty factor and potential anxiety during the experiment. To avoid thermal carry-over effects, a 30-minute break followed the familiarization phase before commencing the first conditioning experiment. Half of the subjects were randomized to warm (all bars 40 °C) and the other half were randomized to cool (all bars 20 °C). On the second experimental day, all subjects underwent TGI conditioning. On day 3, subjects crossed-over into warm and cool conditions.
The study comprised of three different testing days differing only by the conditioning modalities applied. On the first day, all individuals were familiarized with the stimulation devices (CHEPS Stimulator (Pathway, Medoc, RamatYishai, Israel) and customized Thermal Grill device (http://www.sms.hest.ethz.ch/; Engineering Department)) and the temperatures (i.e., neutral, warm, cool, and interlaced cool/warm) that would be used as conditioning modalities. Employing the thermal grill device, all bars were set to either 32 °C (neutral), 20 °C (cool), 40 °C (warm), or alternating 20/40 °C (i.e., the TGI). The individuals were also exposed to three contact heat stimulations delivered by the CHEPS thermode. A 30-minute break followed the familiarization phase upon the commencement of the first experimental session to avoid thermal carry-over effects. The experiment consisted of three measurement blocks of combined conditioning and contact heat stimulation separated by breaks of 5 minutes. For the first two blocks, neutral (32 °C) was chosen as the conditioning modality, while either warm (W), cool (C), or interlaced cool/warm (TGI) (i.e., pseudo-randomized order over the three days: C/TGI/W or W/TGI/C) was presented in the third block. Each block was initiated with a 30 s exposure to the conditioning modality (i.e., neutral, cool, warm, or interlaced cool/warm) by placing the left hand on the thermal grill. Subsequently, individuals were instructed to keep the left hand on the thermal grill device, to fix on a point on the ceiling with their eyes, and to remain relaxed and quiet while recording of the 15 contact heat evoked potential stimulations (CHEPS) applied on the contralateral right hand. All contact heat stimulations were made from a baseline temperature of 42 °C to a peak temperature of 52 °C. The nominal heating rate was 70 °C/s and the cooling rate was 40 °C/s.
During the experiment, subjects were lying in a supine position with eyes open. The experiment consisted of three measurement blocks of combined conditioning and contact heat stimulation separated by 5-minute rest breaks. The first two blocks of contact heat stimulation were delivered to the right hand during neutral (32 °C) conditioning. In a previous study, we observed significant reductions in CHEPs following repetitive stimulation in the same cutaneous area35. Based on these observations, we applied two neutral conditions to establish a stable CHEPs baseline from which to measure changes during conditioning. Warm, cool, or interlaced cool/warm (TGI) was presented in the third block to the left hand. Each block was initiated with a 30 s exposure to the conditioning modality (i.e., neutral, cool, warm, or interlaced cool/warm) by placing the left hand on the thermal grill stimulator. Subjects were instructed to keep the left hand on the thermal grill device, to fix on a point on the ceiling with their eyes, and to remain relaxed and quiet while recording of the CHEPs. Examiners noted if subjects withdrew their hand entirely from the thermal grill for all of the conditions. A total of 15 contact heat stimuli were delivered at an interpulse interval of 8 to 12 seconds. Individuals were asked to rate the perceived intensity of each stimulus from 0 (no pain) to 10 (most unbearable pain) two seconds after stimulation in response to an audio cue. To reduce receptor fatigue or sensitization by overheating of the skin36, the thermode was slightly repositioned following each stimulus within the right C6 dermatome (an area of approximately 4 × 4 cm).
Stimulating device and recording
Responses to noxious stimuli were examined using a contact heat stimulator (Pathway, Medoc, RamatYishai, Israel). The thermode surface (diameter: 27 mm) consists of a heating thermo-foil covered with a layer of thermos-conductive plastic. The nominal heating rate of this device is 70 °C/s (thermo-foil), with a cooling rate of 40 °C/s (peltier element). From peak temperature, the thermode immediately returned to baseline. All measurements of contact heat stimulation were made from a baseline temperature of 42 °C to a peak temperature of 52 °C37, yielding a pulse duration of approximately 393 ms (baseline to peak temperature = 143 ms, peak temperature to baseline = 250 ms).
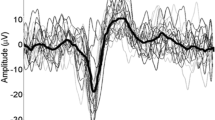
CHEPs were recorded with 9 mm Ag/AgCl surface disc electrodes filled with conductive adhesive gel. Scalp recording sites were prepared with Nuprep (D.O. Weaver & Co. Aurora, CO) and alcohol. In brief, CHEPs are an electrophysiological approach to explore cortical responses to A-delta afferents activated by rapid heat stimuli delivered peripherally. The early CHEPs component (N1) represents a direct measure of nociceptive input at the primary somatosensory cortex38,39. The late waveform (N2P2) reflects later stage processing of the noxious stimulus in the anterior cingulate gyrus and secondary somatosensory cortex38,39. Cortical recording electrodes were positioned according to the International 10–20 system based on available guidelines40. N2P2 was acquired from an active vertex recording electrode (Cz) referenced to linked earlobes (A1-A2)41,42,43,44,45,46. A contralateral temporal active recording electrode (Tc) referenced to Fz was used to acquire N1 as described previously37. All signals were sampled at 2000 Hz using a preamplifier (20000x, bandpass filter 1–300 Hz, ALEA Solutions, Zurich, Switzerland). Data were recorded with 100 ms pre-trigger and a one second post-trigger in a LabView based program (V1.43 CHEP, ALEA Solutions, Zurich, Switzerland). The N2P2 and N1 waveforms were visually detected based on the average of the 15-recorded trials.
Thermal Grill device
A customized thermal grill device was built by Sensory-motor system laboratory at the Swiss Federal Institute of Technology (ETH) Zurich (http://www.sms.hest.ethz.ch/; Engineering Department). The device is equipped with 10 bars (bar dimensions: length 99 mm, diameter 13 mm) each consisting of three Peltier elements, all of which capable of dynamic cooling and heating within a minimum range between 18 and 42 °C. All thermoelectric bars were individually controlled by LabVIEW (LV) software (LV2010, Austin, TX, USA). The software was developed at the Spinal Cord Injury Center (Balgrist University Hospital, Zurich, Switzerland).
Statistical analysis
The statistical analysis focused on addressing changes in CHEP outcomes (rating and N1/N2P2 amplitude) from the neutral condition to warm, cool, and interlaced cool/warm conditioning. “Neutral” CHEPs outcomes were examined to determine their distribution (i.e., normality). Based on an evaluation of skewness and kurtosis parametric statistics were planned. An unstructured linear mixed effects model was applied to examine the main effect of repeated stimulation (second neutral and conditioned) and interaction effect with different condition modalities (warm, cool, interlaced warm and cool). The advantage of the LMM is that one can account for missing data when analyzing longitudinal data47. From this model, we were most interested to determine if conditioning differentially impacted CHEPs outcomes (i.e., interaction effect). In the case of a significant interaction effect, pair-wise comparisons (t-tests) were planned to examine changes in the corresponding CHEPs outcome.
We next considered the relationship between rating of unpleasantness and changes in CHEP outcomes during TGI conditioning. Changes in N1/N2P2 amplitude and pain rating (from the second neutral condition to TGI conditioning) were examined using separate analysis of variance (ANOVA) for N1 and N2P2. The effect of unpleasantness was first examined by way of dichotomizing subjects as “responders” and “non-responders”. TGI responders were defined as any subject that reported the TGI as more unpleasant than warm and cool conditions. Non-responders perceived the TGI as equally or less unpleasant than warm or cool conditioning. Responders/Non-responders was treated as a between subject factor in the models. In order to further explore the relationship between perception and changes in CHEPs outcomes, a second ANOVA explored the effect of TGI unpleasantness as a covariate.
All statistical testing was performed in SPSS (V. 23) and statistical significance was set at p < 0.05.
Results
Subjects
Out of 32 healthy subjects enrolled in the study 4 had to be excluded due to: 1) technical problems during data acquisition (n = 2) and 2) intolerance of the contact heat stimuli applied (n = 2). The remaining 28 subjects comprised of 11 men and 17 women (mean age: 29.4 ± 6.3 years). The mean score of the PCS questionnaire was 11.9 ± 7.7. Participants’ characteristics are summarized in Table 1.
Perception of neutral, cool, warm, and interlaced cool/warm
During exposure to the neutral condition (32 °C), no unpleasantness or pain was reported. The cool modality was reported to be unpleasant by five individuals (NRSunpleasantness = 3.2 ± 2.8) and painful by one individual (NRSpain = 2 ± 0). In response to warm conditioning, four individuals reported unpleasantness (NRSunpleasantness = 1 ± 0) and one pain (NRSpain = 1). The descriptors used for cool stimulation were cool (n = 18), cold (n = 9), and freezing (n = 1). The warm modality was described as warm (27) and burning (1). A total of 13 individuals indicated the interlaced application of cool/warm to be unpleasant (NRSunpleasantness = 3.7 ± 2.1) (summarized in Table 1). The descriptors used by the individuals during the TGI were unpleasant (n = 13), weird (n = 6), confusing (n = 5), hot (n = 4), burning (n = 4), painful (n = 3), and irritating (n = 2). Fourteen individuals spontaneously withdrew their hand in relation to the TGI. All individuals who initially withdraw were able to return their hand for measurement of CHEPs. None of the subjects withdrew their hand to warm or cool conditions. Overall, 10 subjects reported higher unpleasantness during the TGI compared to warm and/or cool conditions (i.e., TGI responders).
Effects of heterotopic conditioning on CHEPs (warm, cool, TGI)
Summary N1 and N2P2 amplitudes, latencies for N1 as well as N2 and P2, and pain ratings of three different conditions (i.e., warm, cold, and TGI) are presented in Table 2.
N2P2 amplitude was significantly affected by the heterotopic conditioning stimulation (main effect: df = 23.7, F = 23.70, p < 0.001), which depended on the conditioning modality (interaction effect: df = 27.3, F = 9.16, p < 0.001). Significant decreases were observed between during warm (t = 6.02, p < 0.001) and cool conditioning (t = 4.97, p < 0.001). In contrast, N2P2 amplitude was significantly increased during interlaced warm and cool conditioning (t = 3.05, p = 0.005) (Fig. 2).
There was no significant effect of repeated stimulation on N1 amplitude (main effect: df = 20.0, F = 0.02, p = 0.89). The modulation of N1 amplitude was, however, dependent on the conditioning modality (interaction effect: df = 19.4, F = 8.32, p = 0.002). During cool conditioning, N1 amplitude significantly decreased (t = 2.52, p = 0.02). While warm conditioning had no significant effect (t = 0.14, p = 0.892), TGI conditioning significantly increased N1 amplitude (t = 2.54, p = 0.021) (Fig. 2). There was no main effect of stimulation or interaction effect for ratings to CHEP stimulation.
Relationship between TGI unpleasantness and changes in N1/N2P2 amplitude
“Responders” and “non-responders” yielded similar changes in N1 (df = 18, F = 0.017, p = 0.899) and N2P2 amplitudes (df = 26, F = 0.911, p = 0.349). As a covariate, there was no effect of “unpleasantness” rating on N1 (df = 18, F = 1.148, p = 0.298) or N2P2 amplitude (df = 26, F = 2.721, p = 0.111).
Discussion
In the 100-year history of the TGI, research has focused primarily on establishing the quality of sensation unmasked by stimulating with interlaced, innocuous warm and cool stimuli3,5,6,48. Others have examined factors that influence the perception of unpleasantness resulting from the thermal grill (e.g., spatial representation)7,49,50,51. In the present study, we applied the TGI as a heterotopic conditioning stimulus and revealed the discrete facilitation of cortical responses to noxious afferent input.
Several lines of converging evidence indicate an important distinction between early and late CHEP waveforms, whereby N1 represents a direct measure of nociceptive input and activation in the primary somatosensory cortex, and N2 and P2 represents later stage processing in the ACC and secondary somatosensory cortex38,52,53,54. Based on this physiological distinction, CHEPs have the potential to differentiate modulation occurring at different levels along the neuroaxis. Cortical modulation has been proposed based on reduced N2P2 amplitude in the absence of N1 changes (e.g., placebo analgesia)55. In contrast, changes in N1 and N2P2 amplitude have been purported as evidence of subcortical modulation (e.g., caloric vestibular stimulation and touch)56,57.
Based on increases in both N1 and N2P2 amplitude during TGI conditioning, it is intriguing to consider that responses to noxious heat were modulated sub-cortically. One potential explanation is that modulation occurs at the spinal level. Previous investigations have demonstrated increases in nociception during unpleasant auditory22,58, visual12,15,23,24,59, and olfactory conditioning60. Closely related to our observations, unpleasant visual stimuli have been shown to increase perception and the amplitude of electrical evoked pain related potentials23. In line with facilitation of N1 and subcortical modulation, the nociceptive flexion reflex is enhanced by unpleasant visual conditioning12,24,59,61,62,63. The facilitation of unpleasant visual stimuli at the spinal level has been attributed to activation in the amygdala, which projects to structures involved in descending control64.
Interestingly, however, TGI related unpleasantness did not have a significant effect on the magnitude of changes in CHEPs – that is, TGI responders demonstrate increases comparable to non-responders. In our study, 10/28 healthy subjects described the TGI as more unpleasant than warm and cool conditioning (responders = 36%). This number is somewhat lower than previous studies3, and may be related to differences in stimulation protocol (e.g., 40/16 °C versus 40/20 °C), how unpleasantness was assessed, and/or variable pressures that subjects manually applied to the thermal grill.
The lack of a relationship between unpleasantness and the facilitation of CHEPs may be a function of underpowered statistics (i.e., only 10 subjects reporting TGI as distinctly unpleasant). More generally, problems may arise from defining “responders”. In the current study, a responder had to identify the TGI as more unpleasant than either warm or cool conditions. Some subjects did not report TGI as “unpleasant” but still physically removed their hand during presentation with interlaced warm and cool bars. This reflexive motor response could be construed, at some level, as evidence of discomfort or unpleasantness associated with the thermal grill. Alternatively, withdrawal (and other descriptors commonly applied, e.g., “weird”) may reflect the TGI as a “metaesthesic” sensation65 – that is, sensation before pain, which is sometimes (but not always) described as unpleasant2. At the core of the problem are confounding variables that contribute to whether an individual reports the thermal grill as unpleasant. Regardless, the ubiquitous facilitation of CHEPs suggests that processes (e.g., central disinhibition) underlying the TGI are independent of subjective perception.
Future directions and limitations
The current study was designed with all subjects undergoing the TGI on the second day of testing. Subjects were randomly assigned to cool or warm for the first session, and crossed into the other condition at the third session. This design was intended to overcome the problem that varying the time from the original familiarization period could influence future responses to the TGI. One potential problem with this approach is that the conditioning effect of TGI on CHEPs may depend on whether the warm or the cool condition came first. We also cannot rule out that the “TGI-effect” on CHEPs was related to being tested on day 2.
Another limitation is that we did not specifically assay changes in arousal. Previous studies demonstrate a strong attention/vigilance component for N2P2, and, to a lesser extent, N139,66,67,68. Increased arousal related to the TGI may then explain, in part, facilitated CHEPs. Increased arousal has been reported in response to the TGI34. In this previous study, greater arousal was related to more unpleasantness34. In order to explain the facilitation of CHEPs, because there was no relationship between unpleasantness and increases in N1 and N2P2 amplitude, the TGI would have had to increase arousal in “non-responders”. Otherwise, the effect of arousal on CHEPs would be bundled into the effect of unpleasantness. Additional investigation is warranted to clarify the effect of the TGI on arousal. Examining cortical responses to other forms of non-noxious stimulation (e.g., electrical) should also be considered to determine if the effect of TGI is specific to noxious stimulation.
Conclusion
In summary, this is the first study providing evidence that heterotopic TGI conditioning facilitates nociceptive evoked potentials. Based on the pattern of facilitation (i.e., both N1 and N2P2), the TGI modulated CHEPs sub-cortically (e.g., spinal cord). Facilitation occurred independently of reported unpleasantness, suggesting that the TGI has a similar pro-nociceptive effect regardless of how it is perceived. Future studies are warranted to further examine the somatosensory conditioning effects of the TGI on cortical responses to afferent stimulation.
Additional Information
How to cite this article: Jutzeler, C. R. et al. Thermal grill conditioning: Effect on contact heat evoked potentials. Sci. Rep. 7, 40007; doi: 10.1038/srep40007 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Leung, A., Shukla, S., Li, E., Duann, J. R. & Yaksh, T. Supraspinal characterization of the thermal grill illusion with fMRI. Mol Pain 10, 18, doi: 10.1186/1744-8069-10-18 (2014).
Bach, P., Becker, S., Kleinbohl, D. & Holzl, R. The thermal grill illusion and what is painful about it. Neurosci Lett 505, 31–35, doi: 10.1016/j.neulet.2011.09.061 (2011).
Lindstedt, F., Lonsdorf, T. B., Schalling, M., Kosek, E. & Ingvar, M. Perception of thermal pain and the thermal grill illusion is associated with polymorphisms in the serotonin transporter gene. PLoS One 6, e17752, doi: 10.1371/journal.pone.0017752 (2011).
Kammers, M. P., de Vignemont, F. & Haggard, P. Cooling the thermal grill illusion through self-touch. Curr Biol 20, 1819–1822, doi: 10.1016/j.cub.2010.08.038 (2010).
Defrin, R., Benstein-Sheraizin, A., Bezalel, A., Mantzur, O. & Arendt-Nielsen, L. The spatial characteristics of the painful thermal grill illusion. Pain 138, 577–586, doi: 10.1016/j.pain.2008.02.012 (2008).
Craig, A. D. & Bushnell, M. C. The thermal grill illusion: unmasking the burn of cold pain. Science 265, 252–255 (1994).
Marotta, A., Ferre, E. R. & Haggard, P. Transforming the thermal grill effect by crossing the fingers. Curr Biol 25, 1069–1073, doi: 10.1016/j.cub.2015.02.055 (2015).
Bouhassira, D., Kern, D., Rouaud, J., Pelle-Lancien, E. & Morain, F. Investigation of the paradoxical painful sensation (‘illusion of pain’) produced by a thermal grill. Pain 114, 160–167, doi: 10.1016/j.pain.2004.12.014 (2005).
Craig, A. D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3, 655–666, doi: 10.1038/nrn894 (2002).
Craig, A. D., Krout, K. & Andrew, D. Quantitative response characteristics of thermoreceptive and nociceptive lamina I spinothalamic neurons in the cat. J Neurophysiol 86, 1459–1480 (2001).
Craig, A. D., Reiman, E. M., Evans, A. & Bushnell, M. C. Functional imaging of an illusion of pain. Nature 384, 258–260, doi: 10.1038/384258a0 (1996).
Bartolo, M. et al. Modulation of the human nociceptive flexion reflex by pleasant and unpleasant odors. Pain 154, 2054–2059, doi: 10.1016/j.pain.2013.06.032 (2013).
Buffington, A. L., Hanlon, C. A. & McKeown, M. J. Acute and persistent pain modulation of attention-related anterior cingulate fMRI activations. Pain 113, 172–184, doi: 10.1016/j.pain.2004.10.006 (2005).
Roy, M., Peretz, I. & Rainville, P. Emotional valence contributes to music-induced analgesia. Pain 134, 140–147, doi: 10.1016/j.pain.2007.04.003 (2008).
Stancak, A. & Fallon, N. Emotional modulation of experimental pain: a source imaging study of laser evoked potentials. Front Hum Neurosci 7, 552, doi: 10.3389/fnhum.2013.00552 (2013).
Yarnitsky, D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 23, 611–615, doi: 10.1097/ACO.0b013e32833c348b (2010).
Nir, R. R. & Yarnitsky, D. Conditioned pain modulation. Curr Opin Support Palliat Care 9, 131–137, doi: 10.1097/SPC.0000000000000126 (2015).
Albu, S., Gomez-Soriano, J., Avila-Martin, G. & Taylor, J. Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain 156, 260–272, doi: 10.1097/01.j.pain.0000460306.48701.f9 (2015).
Millan, M. J. Descending control of pain. Prog Neurobiol 66, 355–474 (2002).
Bushnell, M. C., Ceko, M. & Low, L. A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14, 502–511, doi: 10.1038/nrn3516 (2013).
Wiech, K. & Tracey, I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage 47, 987–994, doi: 10.1016/j.neuroimage.2009.05.059 (2009).
Berna, C. et al. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry 67, 1083–1090, doi: 10.1016/j.biopsych.2010.01.014 (2010).
Kenntner-Mabiala, R. & Pauli, P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology 42, 559–567, doi: 10.1111/j.1469-8986.2005.00310.x (2005).
Rhudy, J. L., Williams, A. E., McCabe, K. M., Rambo, P. L. & Russell, J. L. Emotional modulation of spinal nociception and pain: the impact of predictable noxious stimulation. Pain 126, 221–233, doi: 10.1016/j.pain.2006.06.027 (2006).
Sullivan, M. J. L. B. S. P. J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess, 524–532 (1995).
Meyer, K., Sprott, H. & Mannion, A. F. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res 64, 469–478, doi: 10.1016/j.jpsychores.2007.12.004 (2008).
D’Eon, J. L., Harris, C. A. & Ellis, J. A. Testing factorial validity and gender invariance of the pain catastrophizing scale. J Behav Med 27, 361–372 (2004).
Osman, A. et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 23, 351–365 (2000).
Osman, A. et al. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med 20, 589–605 (1997).
Weissman-Fogel, I., Sprecher, E. & Pud, D. Effects of catastrophizing on pain perception and pain modulation. Experimental brain research 186, 79–85, doi: 10.1007/s00221-007-1206-7 (2008).
Edwards, R. R., Campbell, C. M. & Fillingim, R. B. Catastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain 6, 338–339, doi: 10.1016/j.jpain.2005.02.013 (2005).
Edwards, R. R., Smith, M. T., Stonerock, G. & Haythornthwaite, J. A. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain 22, 730–737, doi: 10.1097/01.ajp.0000210914.72794.bc (2006).
Scheuren, R., Sutterlin, S. & Anton, F. Rumination and interoceptive accuracy predict the occurrence of the thermal grill illusion of pain. BMC Psychol 2, 22, doi: 10.1186/2050-7283-2-22 (2014).
Boettger, M. K., Schwier, C. & Bar, K. J. Sad mood increases pain sensitivity upon thermal grill illusion stimulation: implications for central pain processing. Pain 152, 123–130, doi: 10.1016/j.pain.2010.10.003 (2011).
Haefeli, J., Kramer, J. L., Blum, J. & Curt, A. Heterotopic and homotopic nociceptive conditioning stimulation: distinct effects of pain modulation. Eur J Pain 18, 1112–1119, doi: 10.1002/j.1532-2149.2014.00454.x (2014).
Greffrath, W., Baumgartner, U. & Treede, R. D. Peripheral and central components of habituation of heat pain perception and evoked potentials in humans. Pain 132, 301–311, doi: 10.1016/j.pain.2007.04.026 (2007).
Kramer, J. L., Haefeli, J., Jutzeler, C. R., Steeves, J. D. & Curt, A. Improving the acquisition of nociceptive evoked potentials without causing more pain. Pain 154, 235–241, doi: 10.1016/j.pain.2012.10.027 (2013).
Lee, M. C., Mouraux, A. & Iannetti, G. D. Characterizing the cortical activity through which pain emerges from nociception. J Neurosci 29, 7909–7916, doi: 10.1523/JNEUROSCI.0014-09.2009 (2009).
Mouraux, A. & Iannetti, G. D. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol 101, 3258–3269, doi: 10.1152/jn.91181.2008 (2009).
Cruccu, G. et al. Recommendations for the clinical use of somatosensory-evoked potentials. Clin Neurophysiol 119, 1705–1719, doi: 10.1016/j.clinph.2008.03.016 (2008).
Wydenkeller, S., Wirz, R. & Halder, P. Spinothalamic tract conduction velocity estimated using contact heat evoked potentials: what needs to be considered. Clin Neurophysiol 119, 812–821, doi: 10.1016/j.clinph.2007.12.007 (2008).
Kakigi, R. et al. Human brain processing and central mechanisms of pain as observed by electro- and magneto-encephalography. J Chin Med Assoc 67, 377–386 (2004).
Treede, R. D., Kief, S., Holzer, T. & Bromm, B. Late somatosensory evoked cerebral potentials in response to cutaneous heat stimuli. Electroencephalogr Clin Neurophysiol 70, 429–441 (1988).
Bromm, B. & Chen, A. C. Brain electrical source analysis of laser evoked potentials in response to painful trigeminal nerve stimulation. Electroencephalogr Clin Neurophysiol 95, 14–26 (1995).
Albu, S. et al. Modulation of thermal somatosensory thresholds within local and remote spinal dermatomes following cervical repetitive magnetic stimulation. Neurosci Lett 555, 237–242, doi: 10.1016/j.neulet.2013.06.067 (2013).
Jutzeler, C. R., Curt, A. & Kramer, J. L. Effectiveness of High-Frequency Electrical Stimulation Following Sensitization With Capsaicin. J Pain 16, 595–605, doi: 10.1016/j.jpain.2015.03.005 (2015).
Duricki, D. A., Soleman, S. & Moon, L. D. Analysis of longitudinal data from animals with missing values using SPSS. Nat Protoc 11, 1112–1129, doi: 10.1038/nprot.2016.048 (2016).
Sumracki, N. M., Buisman-Pijlman, F. T., Hutchinson, M. R., Gentgall, M. & Rolan, P. Reduced response to the thermal grill illusion in chronic pain patients. Pain Med 15, 647–660, doi: 10.1111/pme.12379 (2014).
Schaldemose, E. L., Horjales-Araujo, E., Svensson, P. & Finnerup, N. B. Altered thermal grill response and paradoxical heat sensations after topical capsaicin application. Pain 156, 1101–1111, doi: 10.1097/j.pain.0000000000000155 (2015).
Kern, D., Pelle-Lancien, E., Luce, V. & Bouhassira, D. Pharmacological dissection of the paradoxical pain induced by a thermal grill. Pain 135, 291–299, doi: 10.1016/j.pain.2007.12.001 (2008).
Kern, D., Plantevin, F. & Bouhassira, D. Effects of morphine on the experimental illusion of pain produced by a thermal grill. Pain 139, 653–659, doi: 10.1016/j.pain.2008.07.001 (2008).
Carmon, A., Dotan, Y. & Sarne, Y. Correlation of subjective pain experience with cerebral evoked responses to noxious thermal stimulations. Experimental brain research 33, 445–453 (1978).
Baumgartner, U., Tiede, W., Treede, R. D. & Craig, A. D. Laser-evoked potentials are graded and somatotopically organized anteroposteriorly in the operculoinsular cortex of anesthetized monkeys. J Neurophysiol 96, 2802–2808, doi: 10.1152/jn.00512.2006 (2006).
Garcia-Larrea, L., Frot, M. & Valeriani, M. Brain generators of laser-evoked potentials: from dipoles to functional significance. Neurophysiol Clin 33, 279–292 (2003).
Martini, M., Lee, M. C., Valentini, E. & Iannetti, G. D. Intracortical modulation, and not spinal inhibition, mediates placebo analgesia. Eur J Neurosci 41, 498–504, doi: 10.1111/ejn.12807 (2015).
Mancini, F., Beaumont, A. L., Hu, L., Haggard, P. & Iannetti, G. D. Touch inhibits subcortical and cortical nociceptive responses. Pain 156, 1936–1944, doi: 10.1097/j.pain.0000000000000253 (2015).
Ferre, E. R., Haggard, P., Bottini, G. & Iannetti, G. D. Caloric vestibular stimulation modulates nociceptive evoked potentials. Exp Brain Res 233, 3393–3401, doi: 10.1007/s00221-015-4412-8 (2015).
Stancak, A., Ward, H. & Fallon, N. Modulation of pain by emotional sounds: a laser-evoked potential study. Eur J Pain 17, 324–335, doi: 10.1002/j.1532-2149.2012.00206.x (2013).
Rhudy, J. L., Williams, A. E., McCabe, K. M., Nguyen, M. A. & Rambo, P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology 42, 579–587, doi: 10.1111/j.1469-8986.2005.00313.x (2005).
Villemure, C., Slotnick, B. M. & Bushnell, M. C. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain 106, 101–108 (2003).
Rhudy, J. L. & France, C. R. Reliability and validity of a brief method to assess nociceptive flexion reflex (NFR) threshold. J Pain 12, 782–791, doi: 10.1016/j.jpain.2011.01.002 (2011).
Sandrini, G. et al. The lower limb flexion reflex in humans. Prog Neurobiol 77, 353–395, doi: 10.1016/j.pneurobio.2005.11.003 (2005).
Skljarevski, V. & Ramadan, N. M. The nociceptive flexion reflex in humans – review article. Pain 96, 3–8 (2002).
Anders, S., Eippert, F., Weiskopf, N. & Veit, R. The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Soc Cogn Affect Neurosci 3, 233–243, doi: 10.1093/scan/nsn017 (2008).
Procacci, P., Zoppi, M. & Maresca, M. Experimental pain in man. Pain 6, 123–140 (1979).
Beydoun, A., Morrow, T. J., Shen, J. F. & Casey, K. L. Variability of laser-evoked potentials: attention, arousal and lateralized differences. Electroencephalogr Clin Neurophysiol 88, 173–181 (1993).
Le Pera, D., Valeriani, M., Niddam, D., Chen, A. C. & Arendt-Nielsen, L. Contact heat evoked potentials to painful and non-painful stimuli: effect of attention towards stimulus properties. Brain Topogr 15, 115–123 (2002).
Lorenz, J. & Garcia-Larrea, L. Contribution of attentional and cognitive factors to laser evoked brain potentials. Neurophysiol Clin 33, 293–301 (2003).
Acknowledgements
We would like to thank all of the subjects participating in our study. We are grateful for the technical support provided by Michael Herold-Nadig and colleagues from the Sensory-motor system laboratory (http://www.sms.hest.ethz.ch/; Engineering Department at ETH Zurich). The study was supported by the Swiss National Science Foundation (SNF; Grant Nr. 320030 135558) and the Clinical Research Priority Program “Neurorehab” of the University of Zurich, Switzerland. Catherine Jutzeler is an International Research for Paraplegia (IRP) post-doctoral fellow. John Kramer is supported by a Michael Smith Foundation for Health Research and Rick Hansen Scholar Award. Freda Warner is supported by a UBC 4-year fellowship.
Author information
Authors and Affiliations
Contributions
Catherine Jutzeler contributed substantially to the conception and design of the study, the data acquisition, analysis, and interpretation. Furthermore, she drafted the manuscript. Freda Warner was substantially involved in the data collection, data analysis, and revising the manuscript. Johann Wanek was involved in the development of the thermal grill device hardware and software and participated in revising the manuscript. Armin Curt made substantial contributions to study conception and design as well as participated in revising the manuscript critically for important intellectual content. John Kramer contributed substantially to the conception and design of the study, data analysis and interpretation, and was involved in drafting the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Jutzeler, C., Warner, F., Wanek, J. et al. Thermal grill conditioning: Effect on contact heat evoked potentials. Sci Rep 7, 40007 (2017). https://doi.org/10.1038/srep40007
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep40007
- Springer Nature Limited