Abstract
Sperm contain microRNAs (miRNAs), which may have roles in epigenetic control. Regarding phylogenetic relationships among various swine breeds, Yorkshire and Landrace, are considered phenotypically and genetically very similar, but distinctly different from Duroc. The objective of the present study was to compare abundance of boar sperm miRNAs in these three breeds. Overall, 252 prioritized miRNAs were investigated using real-time PCR; relative expression of miRNAs in sperm was similar in Yorkshire and Landrace boars, but significantly different compared to Duroc. Seventeen miRNAs (hsa-miR-196a-5p, hsa-miR-514a-3p, hsa-miR-938, hsa-miR-372-3p, hsa-miR-558, hsa-miR-579-3p, hsa-miR-595, hsa-miR-648, hsa-miR-524-3p, hsa-miR-512-3p, hsa-miR-429, hsa-miR-639, hsa-miR-551a, hsa-miR-624-5p, hsa-miR-585-3p, hsa-miR-508-3p and hsa-miR-626) were down-regulated (P < 0.05; fold regulation ≤−2) in Yorkshire and Landrace sperm, compared to Duroc sperm. Furthermore, three miRNAs (hsa-miR-9-5p, hsa-miR-150-5p, and hsa-miR-99a-5p) were significantly up-regulated in Yorkshire and Landrace sperm compared to Duroc sperm, However, 240 miRNAs were not significantly different (within + 2 fold) between Yorkshire and Landrace sperm. We concluded that miRNAs in sperm were not significantly different between Yorkshire and Landrace boars, but there were significant differences between those two breeds and Duroc boars. Furthermore, integrated target genes for selected down-regulated miRNAs (identified via an in-silico method) appeared to participate in spermatogenesis and sperm functions.
Similar content being viewed by others
Introduction
MicroRNAs are non-coding RNAs that regulate gene expression at a post-transcriptional level and fine-tune expression of ~30% of all mammalian protein-coding genes1. Mature miRNAs are single-stranded, with approximately 22 nucleotides2. MicroRNA genes are substantially regulated (positively or negatively) by many transcription factors and other proteins, in a tissue- or development-specific manner. Similar to protein-coding genes, microRNA genes are transcribed by RNA polymerase II as large primary transcripts (pri-miRNA) and subsequently processed by RNase III enzyme Drosha to form ~70 nucleotide precursor microRNAs (pre-miRNAs). These pre-miRNAs are subsequently transported to the cytoplasm and processed by RNase III enzyme DICER to form mature miRNAs, which are incorporated into a ribonuclear protein to form a miRNA-induced silencing complex (miRISC) that mediates gene silencing3. Post-transcriptional addition of nucleotides to the 3′ ends of pre-miRNAs or mature miRNAs affects miRNA stability or abundance4.
Pig domestication has generated several phenotypically distinct breeds, with large differences among breeds for some traits, including reproduction and meat production5,6,7. Although selection for various environments has resulted in a wide variety of domestic pig breeds with apparently divergent phenotypes8, genetic variability of miRNA expression, which could be linked to post-transcriptional modifications, has not yet been well characterized. However, a few studies reported differential expression (among various pig breeds) of miRNAs in skeletal muscles9, kidneys10 and lungs11. The present investigation proposed that miRNA expression was not only influenced by stages of development within an individual, types of animal tissues, or age, but also by breed.
Sperm concentration, vitality and motility, as well as semen volume in boars, differed significantly among breeds12. For example, Piétrain boars had lower ejaculate volume and total sperm number, but higher sperm concentration than Large White boars13,14. Furthermore, Piétrain boars produced semen with greater volume and total number of sperm, but lower sperm concentration than Duroc boars14,15,16. Despite these known semen traits differences among breeds, differences in miRNAs have not been well characterized. There are indications that expression of sperm miRNAs were altered by environmental changes17 and that sperm traits influenced sperm miRNA expression18. A hallmark study19 identified robust changes in miRNAs in mouse sperm induced by chronic exposure to stress and suggested that transgenerational epigenetic programming was influenced by sperm miRNAs, thereby providing the impetus to elucidate sperm miRNAs and to distinguish breed specificity with regards to level of expression. The objective of the present study was to determine breed differences in sperm miRNA transcriptome among Landrace, Yorkshire and Duroc boars.
Materials and Methods
Ethics statement
This study was performed in strict accordance with standard ethics and use of animal cells for research. The protocol was approved by the institutional animal care and use committee of Washington State University (Protocol Number: 04070-001).
Boars and semen processing
Fresh boar semen was purchased from a commercial boar semen supplier (Swine Genetics International, Cambridge, IA, USA). All boars were fed similar amounts of a common ration, with nutrition and management in accordance with good industry practices. Semen was collected concurrently from all breeds, with all boars 18 to 24 mo old at the time of semen collection.
Sperm-rich fractions from Yorkshire, Landrace and Duroc boars (n = 6 boars per breed) were used. Initial post-collection motility (subjective microscopic examination) was consistently ≥80%. The sperm-rich fraction was diluted in Beltsville Thawing Solution (1:1 volume) and shipped with gel packs (15 °C) to the laboratory by overnight air freight. Upon arrival, sperm motility was ≥70%, with no evidence of immature sperm or somatic cells, based on a subjective microscopic evaluation. Diluted semen (20 mL) was placed in 50-mL Falcon tubes, centrifuged (1000 × g for 20 min at 4 °C) and seminal plasma separated. Sperm were washed twice using Beltsville Thawing Solution (BTS) at 1000 × g for 20 min at 4 °C. Each sperm pellet was re-suspended in BTS at 4 °C, aliquoted into microcentrifuge tubes (~500 × 106 sperm per tube), and centrifuged at 16,000 × g for 10 min at 4 °C. The supernatant was completely removed and the sperm pellet was flash-frozen in liquid nitrogen and stored at −80 °C until used.
RNA purification
Total RNA (contains small RNAs, including miRNAs) was isolated from sperm using an RNeasy plus Universal Mini Kit (Qiagen Inc., Valencia, CA, USA), in accordance with manufacturer’s instructions. Briefly, 900 μL QIAzol Lysis Reagent was added to the sperm pellet (~500 × 106 sperm) and thoroughly homogenized using a disposable homogenizer (Thermo Fisher Scientific, San Francisco, CA, USA). The homogenate was placed at room temperature for 5 min to promote dissociation of nucleoprotein complexes. Then, 100 μL of genomic DNA (gDNA) eliminator solution was added and the mixture shaken vigorously to eliminate contamination by gDNA. Chloroform (180 μL) was then added to the homogenate. After vigorous shaking and 2–3 min incubation at room temperature, the mixture was centrifuged at 12,000 × g at 4 °C for 15 min. After centrifugation, the upper aqueous phase (~600 μL) was transferred to a new microcentrifuge tube and 1.5 times volume of 100% ethanol was added. The mixture was mixed thoroughly by repeated pipetting and the sample was centrifuged in an RNeasy mini spin column (8,000 × g for 15 s at room temperature). The RNA was bound to the membrane of the spin column and subsequently removed using buffer RWT and buffer RPE by centrifugation. Thereafter, RNA was eluted in 60 μL RNase-free water. Purity of the RNA was determined using a Thermo Scientific NanoDrop 1000 spectrophotometer; the ratio of absorbance at 260 and 280 nm, respectively, was ~2.0 for all samples. All RNA samples were stored at −80 °C until used.
Complementary DNA synthesis
Total RNA containing miRNA was used as the starting material. Mature miRNA was reverse-transcribed into cDNA using miScript II RT kit (Qiagen Inc.). Briefly, template RNA was thawed on ice and 10x miScript Nucleics mix, 5x miScript HiSpec buffer and RNase-free water were thawed at room temperature. Reaction components for a 20-μL reaction were 4 μL of HiSpec buffer, 2 μL of Nucleics mix, 2 μL of reverse transcriptase enzyme mix, and 12 μL of RNA template (containing 300 ng RNA plus water). Reverse-transcription reaction components were gently mixed, briefly centrifuged (2000 × g for 10 s) and kept on ice. The mixture was incubated at 37 °C for 60 min and then at 95 °C for 5 min in a Thermocycler (Thermo Fisher Scientific). After incubation, the reaction mix was placed on ice, diluted with 90 μL nuclease-free water, and stored at −20 °C prior to real-time PCR.
Real-time PCR for sperm mature miRNA profiling
Real-time PCR profiling of sperm mature miRNAs (which eliminated the need for validation as required for microarrays) was performed with miScript miRNA PCR arrays, in combination with the miScript SYBR Green PCR Kit, which contains miScript Universal reverse primer and QuantiTect SYBR Green PCR Master Mix. Human miRNome miScript miRNA PCR array 96-well Plates 1, 2 and 3 (Supplementary Tables 1, 2 and 3) were used, as mature miRNAs are conserved between humans and pigs. A sample of miRNA sequences is shown (Table 1). This array profiled expression of 252 most abundantly expressed and well characterized miRNA sequences in boar sperm (hsa-miR-142-5p to hsa-miR-758-3p; Supplementary Tables 1, 2 and 3). A set of controls in the last row of each plate facilitated data analysis using the ΔΔCT method of relative quantification and for assessing performance of reverse transcription and PCR.
The reaction mix for miScript mature miRNA PCR arrays was prepared with 1375 μL of 2x QuantiTect SYBR Green PCR master mix, 275 μL of 10x miScript universal primer, 1000 μL of RNase free water and 100 μL of template cDNA for each 96-well plate. Furthermore, 25 μL of reaction mix was added to each well and template was amplified in a StepOnePlus Real Time PCR system (Applied Biosystems, Foster City, CA, USA). Cycling conditions were an initial heating step at 95 °C for 15 min. Forty cycles included a 15 s denaturation step at 94 °C, a 30 s annealing step at 55 °C and a 30 s extension step at 70 °C. Dissociation curve analysis was done to verify miRNA specificity and identity.
Data analysis
Data analysis was performed using a web-based platform (data analysis center web portal; http://www.qiagen.com). The CT values were uploaded and samples were allocated into a control group (Duroc), Group 1 (Yorkshire) and Group 2 (Landrace), using a sample manager for relative comparison of miRNA expression. The gene RNU6-6P was chosen from the house-keeping gene panel to normalize CT values of target miRNAs, since its CT values had <0.5 cycle variation among samples and breeds. The data quality control page was reviewed to ensure that each sample had acceptable PCR array reproducibility, reverse transcription efficiency, and no genomic DNA contamination. After normalization, analyses were performed. The data overview section was examined for each group’s distribution of CT values and the average of the raw data in each group. Average ∆CT, 2∆CT, Fold Change, and Fold Regulation were calculated and P-values were determined using a Student’s t-test (two-tail distribution and equal variances) on the replicates of 2ΔCT values for each miRNA in each breed group, compared to the control breed group. For all analyses, P < 0.05 and fold regulation on relative comparison ≤−2 or ≥ +2 was considered significant differential expression of miRNA. In addition, Yorkshire (control) and Landrace (group 1) were also compared, using the same parameters.
Integration of target genes
Ten down-regulated miRNAs in comparison groups (Yorkshire/Duroc and Landrace/Duroc) were selected for identification of target genes, which was done with a software algorithm and target mining selection of miRDB (http://mirdb.org/miRDB/mining.html).
Results and Discussion
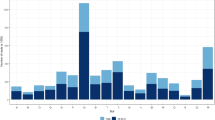
The current investigation elucidated sperm miRNAs in Yorkshire, Landrace and Duroc boars. Abundance of MiRNAs from Yorkshire and Landrace sperm were individually compared to Duroc sperm; thereafter, Yorkshire and Landrace were compared. On a genome analysis of cumulative average of nucleotide diversity, Duroc differed significantly from both Yorkshire and Landrace, whereas the latter two breeds had a very close relationship for nucleotide diversity, consistent with being the two closest breeds of pigs based on phylogenetic analyses20. In that regard, Yorkshire and Landrace shared components with each other20 and based on principal component analyses, could not be clearly distinguished20. This was apparently the first study to demonstrate a lack of differential expression of miRNA in sperm between Yorkshire and Landrace boars, as well as expression differences between these two breeds and Durocs. Real-time PCR array reproducibility, reverse transcription control and reverse transcription efficiency were all satisfactory. More than 50% of miRNAs had <25 threshold cycles in the three breed groups analyzed. Approximately 30% miRNAs from the three groups had 25 to 30 cycles and ~16% miRNAs had 31–35 cycles. Furthermore, >35 cycles was considered as absent calls (Fig. 1), with very few miRNAs in this category.
Out of 252 well-characterized miRNAs investigated in the present study, all were measurably expressed in Duroc, Yorkshire and Landrace sperm. Several miRNA species were down-regulated in Yorkshire and Landrace sperm compared to Duroc sperm, whereas a few miRNAs were up-regulated in Yorkshire and Landrace sperm compared to Duroc sperm (Figs 2 and 3). Furthermore, 27 miRNAs abundances were lower in Yorkshire sperm compared to Duroc sperm (Table 2; <−2 fold regulation and P < 0.05), whereas 30 miRNAs were down-regulated in Landrace boar sperm, compared to Duroc boar sperm (Table 3; <−2 fold regulation and P < 0.05). Seventeen miRNAs (Table 4) were common in significant down-regulation for both comparison groups. Considering fold regulation of +2 as a cut-off, three miRNAs were significantly up-regulated in Yorkshire and Landrace sperm when compared to Duroc sperm (Table 5). It was noteworthy that despite slight variations in miRNA expression between Yorkshire and Landrace, fold regulation of 240 miRNAs did not exceed cut-offs (+ or −2; Table 6). Overall, the 252 miRNAs analyzed in this study were at detectable levels in boar sperm. Although Duroc boars differed from Yorkshire and Landrace boars, the latter two breeds had a close relationship. The down-regulated miRNAs in Yorkshire and Landrace compared to Duroc would have caused up-regulation of genes which may have a role in enhancing desirable semen traits, including greater semen volume, sperm concentration and progressive motility in Yorkshire and Landrace semen compared to Duroc semen. Similar miRNAs expression pattern between Yorkshire and Landrace were consistent with similar quality of semen between these two breeds. Phenotypically similar breeds not only had similar semen traits, but also considerable similarity in sperm miRNA, whereas phenotypically dissimilar breeds differed in semen traits as well as expression of sperm miRNAs. Since all boars used in the study were produced and maintained in the same environment and fed the same ration, differences among breeds in sperm miRNAs were largely attributed to their phenotype and genotype.
Many miRNAs were differentially expressed in mammary gland of lactating Jinhua versus Yorkshire breeds of swine21, whereas several miRNAs expressed in the longissimus dorsi muscle varied between German Landrace and Pietrain pig breeds22. Expression of 125 miRNAs in kidney varied among pig breeds, including the Iberian breed, European Wild Boar ancestor, Landrace, Large White, Piétrain, Meishan and Vietnamese breeds10. In skeletal muscle, 54 miRNAs were differentially expressed in Lantang and Landrace pigs and in adipose tissues, the level of 48 miRNAs varied in Lantang and Landrace pigs9. Furthermore, expression levels for drug metabolizing genes such as SULT1A1, ABCB1, CYP1A2, CYP2E1, CYP3A22 and CYP3A29 differed among Duroc, Landrace, Yorkshire and Hampshire pigs23. In addition, there was higher protein content of Drosha, Dicer and Ago2 (main enzymes required for biogenesis of miRNAs) in the liver of Erhualian versus Large White piglets24. Also, semen quality, including sperm concentration, ejaculate volume and sperm number differed among breeds (Large White, Pietrain and Duroc x Pietrain), along with seasonal changes13. Estimated overall heritabilities for semen volume and sperm concentration were ~0.20, which is considerable25. Specifically, Wolf (2010) calculated the heritability of semen volume as 0.20 +/− 0.019 for Large White and 0.25 +/− 0.018 for Landrace, and the heritability of sperm concentration as 0.18 (SEM = 0.012 and 0.014) in both breeds26. On a comparison of boar sperm output among various breeds (Czech Large White, Czech Landrace, Prestice Black-Pied, Czech Meat Pig, Hampshire, Duroc, Pietrain and Large White), Duroc boars had lowest values for sperm quality, whereas Large White had best sperm quality14. The present study illustrated differential expression of sperm miRNAs in Yorkshires, Landraces and Durocs. Whereas several other studies have demonstrated that Duroc breed differed genetically from Yorkshire and Landrace breeds for various parameters, the present study identified breed differences and similarities in the context of sperm miRNAs.
Boars have been selected for superior genetics; historically this selection focused on production traits, including age and back fat thickness at 100 kg, feed efficiency, lean yield and litter size27. These selection pressures may have negatively affected reproductive traits, including semen quality. However, there is increasing pressure to incorporate male fertility traits, such as sperm number, sperm fertilizing capacity and boar conformation for efficient semen collection. A recent study28 recommended that selection indices include four main semen traits, namely volume, concentration, progressive motility, and morphologically abnormal sperm, without compromising genetic benefits from maternal traits and with a minimal loss of genetic gain from paternal traits (other than semen traits) for economic value. Following the current characterization of breed differences in sperm miRNAs, the logical next step would be to determine associations between sperm miRNA abundance and common traits, including progressive motility and fertilizing capacity of sperm. Based on the outcomes, it may be appropriate to use sperm miRNA abundance as a trait in boar selection.
Since the primary function of miRNAs is post-transcriptional regulation of expression of target genes, this study also identified target genes for some down-regulated miRNAs in Yorkshire and Landrace boar sperm, compared to Duroc boar sperm. Target genes with the highest target score (≥90) are shown (Table 7). Several target genes, including SLC9A6, AQP4, EGFR, MAP3K1, NR2C2, PTPRG, RET, ABCB9, PTEN and JAM229,30,31,32,33,34,35,36,37,38 are involved in either spermatogenesis or sperm function. We inferred that these target genes would have been up-regulated in Yorkshire and Landrace sperm, compared to Duroc sperm. Some of these target genes have been associated with spermatogenesis and sperm function. Therefore, down-regulated miRNAs and their target genes presumably contributed to differences in semen quality between phenotypically distinct breeds.
Conclusions
In summary, all 252 miRNAs analyzed were detected in sperm of Yorkshire, Landrace and Duroc boars, with potential roles in epigenetic regulation of sperm function. Abundance of sperm miRNAs varied among breeds; Duroc boars differed from Yorkshire and Landrace boars, whereas the latter two breeds had a close relationship.
Additional Information
How to cite this article: Kasimanickam, V. and Kastelic, J. MicroRNA in sperm from Duroc, Landrace and Yorkshire boars. Sci. Rep. 6, 32954; doi: 10.1038/srep32954 (2016).
References
Sevignani, C., Calin, G. A., Siracusa, L. D. & Croce, C. M. Mammalian microRNAs: a small world for fine-tuning gene expression. Mamm Genome 17, 189–202 (2006).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Krol, J., Loedige, I. & Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 11, 597–610 (2010).
Winter, J., Jung, S., Keller, S., Gregory, R. I. & Diederichs, S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11, 228–234 (2009).
Amaral, A. J. et al. Genome-wide footprints of pig domestication and selection revealed through massive parallel sequencing of pooled DNA. PloS One 6, e14782 (2011).
Ojeda, A. et al. Evolutionary study of a potential selection target region in the pig. Heredity 106, 330–338 (2011).
Gao, Y. et al. Detection of differentially expressed genes in the longissimus dorsi of northeastern indigenous and large white pigs. Genetics and Molecular Research: GMR 10, 779–791 (2011).
Porter, V. & Tebbit J. Pigs. A handbook of the breeds of the world. (Mountfield: Helm, East Sussex, 1993).
Li, H. Y. et al. Identification and comparison of microRNAs from skeletal muscle and adipose tissues from two porcine breeds. Anim Genet 43, 704–713 (2012).
Timoneda, O. et al. miRNA expression profile analysis in kidney of different porcine breeds. PLoS One 8, e55402 (2013).
Li, J. et al. Difference in microRNA expression and editing profile of lung tissues from different pig breeds related to immune responses to HP-PRRSV. Sci Rep 5, 9549 (2015).
Knecht, D., Środoń, S. & Duziński, K. The influence of boar breed and season on semen parameters. South African Journal of Animal Science 44, 1–9 (2014).
Ciereszko, A., Ottobre, J. S. & Glogowski, J. Effects of season and breed on sperm acrosin activity and semen quality of boars. Anim Reprod Sci 64, 89–96 (2000).
Smital, J., De Sousa, L. L. & Mohsen, A. Differences among breeds and manifestation of heterosis in AI boar sperm output. Anim Reprod Sci 80, 121–130 (2004).
Kondracki, S., Wysokińska, A., Banaszewska, D. & Zajda, J. Breed-related variation in ejaculate traits of AI boars. Anim Sci Pap Rep 24, 121–129 (2006).
Smital, J. Effects influencing boar semen. Anim Reprod Sci 110, 335–346 (2009).
Stowe, H. M. et al. The bull sperm microRNAome and the effect of fescue toxicosis on sperm microRNA expression. PLoS One 9, e113163 (2014).
Curry, E., Safranski, T. J. & Pratt, S. L. Differential expression of porcine sperm microRNAs and their association with sperm morphology and motility. Theriogenology 76, 1532–1539 (2011).
Rodgers, A. B., Morgan, C. P., Bronson, S. L., Revello, S. & Bale, T. L. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J Neurosci 33, 9003–9012 (2013).
Kim, H. et al. Exploring the genetic signature of body size in Yucatan miniature pig. PLoS One 10, e0121732 (2015).
Peng, J., Zhao, J. S., Shen, Y. F., Mao, H. G. & Xu, N. Y. MicroRNA expression profiling of lactating mammary gland in divergent phenotype swine breeds. Int J Mol Sci 16, 1448–1465 (2015).
Siengdee, P. et al. Pre- and post-natal muscle microRNA expression profiles of two pig breeds differing in muscularity. Gene 561, 190–198 (2015).
Howard, J. T., O’Nan, A. T., Maltecca, C., Baynes, R. E. & Ashwell, M. S. Differential gene expression across breed and sex in commercial pigs administered fenbendazole and flunixin meglumine. PLoS One 10, e0137830 (2015).
Li, R., Jia, Y., Zou, H. & Zhao, R. Breed-specific expression of DROSHA, DICER and AGO2 is regulated by glucocorticoid-mediated miRNAs in the liver of newborn piglets. Anim Genet 45, 817–826 (2014).
Wolf, J. Genetic parameters for semen traits in AI boars estimated from data on individual ejaculates. Reprod Domest Anim 44, 338–344 (2009).
Wolf, J. Heritabilities and genetic correlations for litter size and semen traits in Czech Large White and Landrace pigs. J Anim Sci 88, 2893–2903 (2010).
Robinson J. A. & Buhr M. M. Impact of genetic selection on management of boar replacement. Theriogenology 63, 668–678 (2005).
González-Peña, D., Knox, R. V., MacNeil, M. D. & Rodríguez-Zas, S. L. Genetic gain and economic values of selection strategies including semen traits in three- and four-way crossbreeding systems for swine production. J Anim Sci 93, 879–891 (2015).
Fuster, D. G. & Alexander, R. T. Traditional and emerging roles for the SLC9 Na+/H+ exchangers. Pflugers Arch 466, 61–76 (2014).
Cho, Y. S., Svelto, M. & Calamita, G. Possible functional implications of aquaporin water channels in reproductive physiology and medically assisted procreation. Cell Mol Biol (Noisy-le-grand) 49, 515–519 (2003).
Michailov, Y., Ickowicz, D. & Breitbart, H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev Biol 396, 246–255 (2014).
Thundathil, J., de Lamirande, E. & Gagnon, C. Different signal transduction pathways are involved during human sperm capacitation induced by biological and pharmacological agents. Mol Hum Reprod 8, 811–816 (2002).
Mu, X. et al. Targeted inactivation of testicular nuclear orphan receptor 4 delays and disrupts late meiotic prophase and subsequent meiotic divisions of spermatogenesis. Mol Cell Biol 24, 5887–5899 (2004).
González-Fernández, L. et al. Identification of protein tyrosine phosphatases and dual-specificity phosphatases in mammalian spermatozoa and their role in sperm motility and protein tyrosine phosphorylation. Biol Reprod 80, 1239–1252 (2009).
Tyagi, G. et al. Loss of Etv5 decreases proliferation and RET levels in neonatal mouse testicular germ cells and causes an abnormal first wave of spermatogenesis. Biol Reprod 81, 258–266 (2009).
Melaine, N. et al. Molecular cloning of several rat ABC transporters including a new ABC transporter, Abcb8, and their expression in rat testis. Int J Androl 29, 392–399 (2006).
Huang, Y., Mao, X., Boyce, T. & Zhu, G. Z. Dispensable role of PTEN in mouse spermatogenesis. Cell Biol Int 35, 905–908 (2011).
Zhang, X. & Lui, W. Y. Transforming growth factor-β3 regulates cell junction restructuring via MAPK-mediated mRNA destabilization and Smad-dependent protein degradation of junctional adhesion molecule B (JAM-B). Biochim Biophys Acta 1849, 601–611 (2015).
Acknowledgements
Authors gratefully acknowledge financial support for this study provided by the College of Veterinary Medicine, Washington State University.
Author information
Authors and Affiliations
Contributions
V.K. designed the study, performed the experiment, analyzed the data and drafted the manuscript. J.K. analyzed the data and revised the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Kasimanickam, V., Kastelic, J. MicroRNA in sperm from Duroc, Landrace and Yorkshire boars. Sci Rep 6, 32954 (2016). https://doi.org/10.1038/srep32954
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep32954
- Springer Nature Limited