Abstract
Osteoprotegerin (OPG), receptor activator of nuclear factor-ΚB ligand (RANKL) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) have been involved in rheumatoid arthritis (RA) pathophysiology. In this study, we assessed messenger RNA (mRNA) expression of these molecules by qPCR in peripheral blood from 26 patients with RA (12 of them with ischemic heart disease –IHD) and 10 healthy controls. Correlation coefficients between OPG, RANKL and TRAIL expression levels in RA patients and their clinical and demographic characteristics were also evaluated. Whereas OPG and OPG/TRAIL ratio expression were significantly increased in RA patients compared to controls (fold change = 1.79, p = 0.013 and 2.07, p = 0.030, respectively), RANKL/OPG ratio was significantly decreased (fold change = 0.50, p = 0.020). No significant differences were found between patients and controls in RANKL and TRAIL expression. Interestingly, TRAIL expression was significantly higher in RA patients with IHD compared to those without IHD (fold change = 1.46, p = 0.033). Moreover, biologic disease-modifying antirheumatic drugs (DMARDs) significantly decreased RANKL expression in RA patients (p = 0.016). Our study supports an important role of OPG and TRAIL in RA. Furthermore, it highlights an effect of biologic DMARDs in the modulation of RANKL.
Similar content being viewed by others
Introduction
Osteoprotegerin (OPG)/receptor activator of nuclear factor-ΚB ligand (RANKL)/tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) system has been involved in the pathophysiology of rheumatoid arthritis (RA)1,2. OPG is a soluble glycoprotein, member of the TNF-receptor superfamily and an inhibitor of two cytokines belonging to the TNF ligand family, RANKL and TRAIL3. RANKL functions as a key factor for osteoclastogenesis, since the binding with its receptor, receptor activator of nuclear factor-ΚB (RANK), favors the activation of osteoclasts and bone resorption. OPG reduces RANKL-RANK interactions and thus inhibits osteoclastogenesis3. In this regard, RANKL/OPG ratio plays an important role regulating bone homeostasis in RA, a disease characterized by bone and cartilage destruction1,4. Furthermore, a protective role in RA has been shown for TRAIL, an anti-inflammatory molecule mainly known as inductor of apoptosis in tumor cells. Thus, TRAIL could be involved in the regulation of the systemic inflammatory autoimmune response produced by the disease2. However, increasing evidence suggest it could be a pleiotropic cytokine with a dual function5. Since OPG can bind to TRAIL inhibiting their function, it has been proposed that the OPG/TRAIL ratio could also be involved in RA pathogenesis6.
It is well known that RA patients have an accelerated atherosclerotic process, leading to an increased risk of cardiovascular (CV) disease, including ischemic heart disease (IHD)7. In this sense, OPG/RANKL/TRAIL system has also been related to atherosclerosis and CV disease8. Due to the important role that these molecules play in CV disease, they have been proposed as potential biomarkers of CV disease8,9.
Previously, our group reported that OPG serum levels were associated with CV disease in RA patients10. Therefore, to confirm the role of OPG and its ligands, RANKL and TRAIL, in the pathophysiology of RA, we determined for the first time the differential gene expression of these molecules in peripheral blood from patients with RA, with and without IHD and healthy controls. Changes in expression level of these molecules and its correlation with clinical and demographic characteristics of RA patients will also be useful to elucidate this role.
Taking into account that both RA and CV disease are inflammatory systemic diseases, the assessment of OPG, RANKL and TRAIL expression at a systemic level (blood) is of main importance.
Patients and Methods
Patients and controls
For experiments involving humans and the use of human blood samples, all the methods were carried out in accordance with the approved guidelines and regulations, according to the Declaration of Helsinki. All experimental protocols were approved by the Ethics Committee of clinical research of Cantabria (CEIC-C, Number of reference 14/2012). Informed consent was obtained from all subjects.
Twenty-six RA patients, 12 of them with IHD and ten healthy controls matched for age and sex were recruited from Hospital Universitario Marqués de Valdecilla (Santander, Cantabria, Spain). All RA patients met the 2010 American College of Rheumatology classification criteria for RA11. We recorded the main demographic and clinical characteristics of patients, measured the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and lipids and calculated the Disease Activity Score in 28 joints (DAS28)-CRP and –ESR at the time of assessment. The presence of IHD and traditional CV risk factors were defined as previously reported10. The characteristics of the RA patients and controls included in this study have previously been described12.
Blood RNA extraction
As we previously described, total RNA was isolated from peripheral blood samples according to the manufacturer’s protocol using NucleoSpin RNA Blood Midi Kit (Macherey-Nagel)12. Samples were concentrated using GeneJET RNA Cleanup and Concentration Micro Kit (Thermo Scientific) and stored at −80 °C until further processing.
Quantitative real-time polymerase chain reaction (PCR)
For each sample, 1 μg of total RNA was reverse transcribed into cDNA using iScriptTM Advanced cDNA Synthesis Kit for RT-qPCR (Bio-Rad, Hercules, CA, USA). 20 ng of cDNA was used for quantitative real-time PCR (qPCR), using SsoAdvancedTM Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA). Primers for amplification of OPG, RANKL, TRAIL, beta-actin and GAPDH were acquired from PrimePCR Assays, Bio-Rad, Hercules, CA, USA. All samples were assayed in triplicate and controls were included in each reaction. qPCRs were performed in a 7900 HT real-time instrument (Applied Biosystems, Foster City, CA, USA) using the following conditions: stage 1: 95 °C for 2 min, stage 2: 95 °C for 5 sec followed by 60 °C for 30 sec repeated for 45 cycles. The threshold cycle (Ct) was manually established and recorded by the SDS 2.2.2 software (Applied Biosystems, Foster City, CA, USA). The relative OPG, RANKL and TRAIL messenger RNA (mRNA) expression was analyzed by the comparative Ct method, using beta-actin and GAPDH as housekeeping genes12. Normalized expression levels were obtained for each sample and the RANKL/OPG and OPG/TRAIL ratios were calculated with these data. Mean values were determined for each study group and fold-change (changed amount of expression) was calculated by comparing the means between different groups. A fold-change value less than 1 indicates negative or down-regulation of the study gene. A fold-change value greater than 1 indicates positive or up-regulation.
OPG, RANKL and TRAIL serum levels by enzyme-linked immunosorbent assay (ELISA)
OPG levels were measured as previously described10. Concentrations of free soluble RANKL and TRAIL were also determined by ELISA, using kits from Biomedica (BI-20462) and R&D Systems (DTRL00), respectively, according to the manufacturer’s instructions. All samples were analyzed in duplicate.
Statistical analysis
Results were expressed as mean ± standard deviation (SD). Normalized expression values and protein levels obtained for each sample were analyzed by GraphPad Prism® 3.0 (GraphPad Software, San Diego, CA, USA) using nonparametric Mann-Withney U test to compare two study groups. A p value < 0.05 was considered statistically significant.
OPG, RANKL and TRAIL expression among patients with RA and controls was first compared by analysis of covariance (ANCOVA), with adjustment for age, sex and traditional CV risk factors.
Partial correlations of demographic and clinical characteristics in RA patients with their relative OPG, RANKL and TRAIL mRNA levels were performed after adjusting for age at the time of the study, sex and classic CV risk factors via estimation of the Pearson partial correlation coefficient (r). The same statistical analysis was conducted to analyze whether OPG expression levels were associated with RANKL and TRAIL mRNA expression. A p value < 0.05 was considered statistically significant. These analyses were performed using Stata 12/SE (StataCorp, College Station, TX).
Results
OPG, RANKL and TRAIL expression in RA
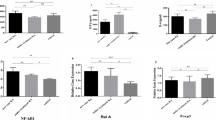
Expression of the OPG gene was significantly increased in RA patients compared to controls (fold change = 1.79, p = 0.013, Table 1). In this regard, patients with RA had a mean OPG expression of 6.68 ± 3.69 whereas controls had a mean OPG expression of 3.74 ± 2.00 (Table 1 and Fig. 1a). However, no significant differences were found between patients and controls in RANKL and TRAIL mRNA expression (Table 1). A decreased RANKL/OPG ratio and an increased OPG/TRAIL ratio was observed in patients compared to controls (fold change = 0.50 and 2.07, respectively). These differences in expression were statistically significant (p = 0.020 and p = 0.030, respectively, Table 1).
Increased OPG mRNA expression in patients with RA and TRAIL mRNA expression in RA patients with IHD.
OPG and TRAIL expression was normalized to two housekeeping genes (beta-actin and GAPDH). (a) Differential expression of relative OPG mRNA was analyzed between control group (n = 10) and RA patients (n = 26). (b) Differential expression of relative TRAIL mRNA was analyzed between RA patients stratified according to the presence (n = 12) or absence of IHD (n = 14). Each bar represents mean value ± SD obtained for each sample in triplicate.
When RA patients were stratified according to the presence or absence of IHD, we disclosed that the relative TRAIL mRNA expression was significantly higher in RA patients with compared to those without IHD (3.58 ± 1.55 vs 2.45 ± 1.19, fold change = 1.46, p = 0.033, Fig. 1b). However, no significant differences were found in OPG and RANKL mRNA levels or in RANKL/OPG and OPG/TRAIL ratios between these two groups (data not shown).
Association of OPG, RANKL and TRAIL mRNA expression with clinical and demographic characteristics in RA patients
An inverse association was found between OPG expression and total cholesterol (r = −0.48, p = 0.040). Additionally, a negative correlation was observed between RANKL expression and CRP (r = −0.52, p = 0.024) and DAS28 CRP (r = −0.56, p = 0.014). Interestingly, RA patients undergoing treatment with biologic disease-modifying antirheumatic drugs (DMARDs) showed a statistically significant decrease of RANKL expression (r = −0.54, p = 0.016). Regarding TRAIL, we disclosed a positive correlation with RA disease duration (r = 0.46, p = 0.047) and CRP (r = 0.58, p = 0.010).
Influence of OPG expression on its ligands
No association was found between relative OPG mRNA expression and RANKL or TRAIL mRNA levels (p = 0.89 and p = 0.85, respectively) in RA patients.
OPG, RANKL and TRAIL serum levels in RA
ELISA was performed in order to provide supplementary information on the OPG, RANKL and TRAIL mRNA expression and the corresponding protein levels. OPG serum levels were significantly increased in RA patients compared to controls (9.06 ± 6.00 vs 3.85 ± 1.51 ng/ml, p = 0.002). No significant differences were found between patients and controls in RANKL and TRAIL concentrations.
Discussion
Several molecules have been implicated in the pathogenesis of both RA and CV disease13. In this regard, the OPG/RANKL/TRAIL system has been linked to bone loss in RA and CV risk1,6,8. To further study the involvement of this system in RA at the gene expression level, we evaluated the OPG, RANKL and TRAIL mRNA levels in peripheral blood from patients with RA and their relationship with clinical and demographical characteristics of RA disease. Furthermore, we studied its potential association with IHD in patients with RA.
Previously, it has been reported that the expression of many cytokines and growth factors is up- or down-regulated in both peripheral blood and local joints in patients with RA14. In this regard, circulating (or peripheral) blood cells from RA patients have been used in previous studies to assess the OPG/RANKL/TRAIL gene expression for prognosis or diagnosis of RA14,15. In addition, considering that RA and CV disease affect multiple tissues at a systemic level, we believe that the assessment of the role of these molecules in circulation is very relevant.
OPG regulates bone homeostasis upon binding to RANKL and also participates in the pathogenesis of atherosclerosis and CV diseases8. We previously described that OPG concentrations are independently associated with endothelial activation, carotid atherosclerosis and established CV disease in RA10,16. In the present study, we observed that the expression of OPG was up-regulated in RA patients, supporting its pivotal role in the pathogenesis of RA. Moreover, the increase in OPG serum levels disclosed in our patients with RA enables us to confirm that the upregulation of OPG at a systemic level leads to a high release of OPG protein in serum. In addition, OPG mRNA levels were inversely associated with total cholesterol. This finding is in line with former results of our group performed in RA and ankylosing spondylitis patients, which disclosed a negative correlation between OPG and total and LDL cholesterol, even if they were assessed regarding serum/plasma concentrations16,17. However, in another study from our group we could not find an association between OPG levels and cholesterol from a different cohort of patients with RA10. These results may appear to be contradictory at first glance. However, we believe that potential associations observed between a clinical characteristic or disease with gene expression at mRNA level does not necessarily have to be reflected in protein concentrations. Nevertheless, further studies with a larger number of samples should be performed to elucidate these discrepancies.
Regarding RANKL mRNA expression, no significant differences were found between our patients and controls. The same finding was previously shown in synovial fluid-neutrophils from RA patients compared to normal blood-neutrophils from healthy individuals18. In addition, we disclosed a negative association between RANKL mRNA and treatment with biologic DMARDs. Therefore, it is plausible to think that RANKL expression is being modulated at a transcriptional level by these drugs. Accordingly, biologic and synthetic DMARDs also decreased RANKL mRNA in fibroblasts from RA patients19,20.
TRAIL is another molecule involved in this system. It has also been associated with atherosclerosis and CV disease2,8. With respect to this, we disclosed a significant up-regulation of this gene in patients with IHD compared to those without IHD. In keeping with this result, TRAIL concentrations were found to be increased in RA patients with heart failure21. Since TRAIL is an anti-inflammatory molecule, the high expression observed in RA patients with IHD could be the result of a compensatory mechanism to limit the potential pro-inflammatory effects triggered by other cytokines. However, depending on the context, the role of TRAIL is still confusing5. Consequently, our data suggest that high expression of TRAIL may be a potential predictor of CV disease in RA patients. In addition, the positive relationship between TRAIL mRNA expression and both CRP concentrations and RA disease duration reinforces our hypothesis. In this regard, CRP, involved in systemic inflammation and CV disease, has also been related to TRAIL serum levels in other chronic inflammatory arthritis, such as psoriatic arthritis22.
Finally, the upregulation of OPG observed in our RA patients led to an increased OPG/TRAIL ratio. Since TRAIL mRNA was associated with IHD in our study, this ratio may also play an important role in the development CV disease. This finding is agreement with Secchiero et al., who disclosed an increased in OPG/TRAIL ratio levels in patients with acute myocardial infarction who developed heart failure23.
Our study supports an important role of OPG and TRAIL in RA. Furthermore, it highlights an effect of biologic DMARDs in the modulation of RANKL.
Additional Information
How to cite this article: Remuzgo-Martínez, S. et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci. Rep. 6, 29713; doi: 10.1038/srep29713 (2016).
References
Geusens, P. The role of RANK ligand/osteoprotegerin in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 4, 225–233 (2012).
Neve, A., Corrado, A. & Cantatore, F. P. TNF-related apoptosis-inducing ligand (TRAIL) in rheumatoid arthritis: what’s new? Clin. Exp. Med. 14, 115–120 (2014).
Vitovski, S., Phillips, J. S., Sayers, J. & Croucher, P. I. Investigating the interaction between osteoprotegerin and receptor activator of NF-kappaB or tumor necrosis factor-related apoptosis-inducing ligand: evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J. Biol. Chem. 282, 31601–31609 (2007).
Vanderborght, A. et al. Osteoprotegerin and receptor activator of nuclear factor-kappaB ligand mRNA expression in patients with rheumatoid arthritis and healthy controls. J. Rheumatol. 31, 1483–1490 (2004).
Audo, R., Combe, B., Hahne, M. & Morel, J. The two directions of TNF-related apoptosis-inducing ligand in rheumatoid arthritis. Cytokine 63, 81–90 (2013).
Castellino, G., Corallini, F., Trotta, F. & Secchiero, P. Is there any role for tumour necrosis factor related apoptosis inducing ligand-osteoprotegerin (TRAIL-OPG) interaction in rheumatoid arthritis? Ann. Rheum. Dis. 67, 1196–1197 (2008).
Gonzalez-Gay, M. A., Gonzalez-Juanatey, C., Vazquez-Rodriguez, T. R., Martin, J. & Llorca, J. Endothelial dysfunction, carotid intima-media thickness and accelerated atherosclerosis in rheumatoid arthritis. Semin. Arthritis. Rheum. 38, 67–70 (2008).
Venuraju, S. M., Yerramasu, A., Corder, R. & Lahiri, A. Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J. Am. Coll. Cardiol. 55, 2049–2061 (2010).
Bernardi, S., Milani, D., Fabris, B., Secchiero, P. & Zauli, G. TRAIL as biomarker and potential therapeutic tool for cardiovascular diseases. Curr. Drug Targets 13, 1215–1221 (2012).
López-Mejias, R. et al. Osteoprotegerin concentrations relate independently to established cardiovascular disease in rheumatoid arthritis. J. Rheumatol. 42, 39–45 (2015).
Aletaha, D. et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 62, 2569–2581 (2010).
Remuzgo-Martínez, S. et al. Decreased expression of methylene tetrahydrofolate reductase (MTHFR) gene in patients with rheumatoid arthritis. Clin. Exp. Rheumatol. 34, 106–110 (2016).
Rodríguez-Rodríguez, L. et al. Genetic markers of cardiovascular disease in rheumatoid arthritis. Mediators Inflamm. 2012, 574817 (2012).
Grcevic, D. et al. Peripheral blood expression profiles of bone morphogenetic proteins, tumor necrosis factor-superfamily molecules and transcription factor Runx2 could be used as markers of the form of arthritis, disease activity and therapeutic responsiveness. J. Rheumatol. 37, 246–256 (2010).
Ziolkowska, M. et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis. Rheum. 46, 1744–1753 (2002).
Dessein, P. H. et al. Independent relationship of osteoprotegerin concentrations with endothelial activation and carotid atherosclerosis in patients with severe rheumatoid arthritis. J. Rheumatol. 41, 429–436 (2014).
Genre, F. et al. Osteoprotegerin correlates with disease activity and endothelial activation in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clin. Exp. Rheumatol. 32, 640–646 (2014).
Poubelle, P. E., Chakravarti, A., Fernandes, M. J., Doiron, K. & Marceau, A. A. Differential expression of RANK, RANK-L and osteoprotegerin by synovial fluid neutrophils from patients with rheumatoid arthritis and by healthy human blood neutrophils. Arthritis Res. Ther. 9, R25 (2007).
Lee, C. K. et al. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor kappaB, osteoprotegerin and receptor activator of nuclear factor kappaB ligand. Arthritis Rheum. 50, 3831–3843 (2004).
Wei, Y. et al. Inhibitory Effect of a Novel Antirheumatic Drug T-614 on the IL-6-Induced RANKL/OPG, IL-17 and MMP-3 Expression in Synovial Fibroblasts from Rheumatoid Arthritis Patients. Biomed Res. Int. 2015, 214683 (2015).
Dessein, P. H. et al. TNF-related apoptosis-inducing ligand and cardiovascular disease in rheumatoid arthritis. Clin. Exp. Rheumatol. 33, 491–497 (2015).
Hofbauer, L. C., Schoppet, M., Christ, M., Teichmann, J. & Lange, U. Tumour necrosis factor-related apoptosis-inducing ligand and osteoprotegerin serum levels in psoriatic arthritis. Rheumatology (Oxford) 45, 1218–1222 (2006).
Secchiero, P. et al. An imbalanced OPG/TRAIL ratio is associated to severe acute myocardial infarction. Atherosclerosis 210, 274–277 (2010).
Acknowledgements
We wish to thank all the patients with RA and controls that participated to make this study possible. We want to specially thank Jesús González Vela and Patricia Fuentevilla for their technical assistance. This study was supported by European Union FEDER funds and “Fondo de Investigación Sanitaria” (grant PI12/00060 and PI15/00525) from “Instituto de Salud Carlos III” (ISCIII) (Spain). This work was also partially supported by RETICS Programs RD12/0009 (RIER) from ISCIII (Spain). FG is a recipient of a Sara Borrell postdoctoral fellowship from the “ISCIII” (CD15/00095). RL-M and BU are supported by funds from the RETICS Program (RIER) (RD12/0009/0013).
Author information
Authors and Affiliations
Contributions
S.R.-M., F.G. and R.L.-M. carried out the conception and design of the study, expression assays, data and statistical analysis and helped to draft the manuscript. B.U., V.M., T.P., A.C., R.B. and J.M. participated in expression assays, acquisition of clinical data and samples and helped to draft the manuscript. J.L. and M.A.G.-G. have contributed to the conception and design of the study, analysis and interpretation of data, coordination, helped to draft the manuscript and have given final approval of the version to be published.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Remuzgo-Martínez, S., Genre, F., López-Mejías, R. et al. Expression of osteoprotegerin and its ligands, RANKL and TRAIL, in rheumatoid arthritis. Sci Rep 6, 29713 (2016). https://doi.org/10.1038/srep29713
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep29713
- Springer Nature Limited
This article is cited by
-
Osteopontin, osteoprotegerin and musculoskeletal ultrasound findings in first-degree relatives of rheumatoid arthritis: potential markers of preclinical disease
BMC Musculoskeletal Disorders (2024)
-
Fractionated whole body γ-irradiation aggravates arthritic severity via boosting NLRP3 and RANKL expression in adjuvant-induced arthritis model: the mitigative potential of ebselen
Inflammopharmacology (2023)
-
Assessment of bone metabolism biomarkers in serum and saliva of cirrhotic patients
Clinical Oral Investigations (2022)
-
Dual energy CT in musculoskeletal applications beyond crystal imaging: bone marrow maps and metal artifact reduction
Skeletal Radiology (2022)
-
Cannabinoid-based therapy as a future for joint degeneration. Focus on the role of CB2 receptor in the arthritis progression and pain: an updated review
Pharmacological Reports (2021)