Abstract
Background
The inflammasome plays an important role in rheumatoid arthritis (RA), which has rarely been systematically reported. The aim of this study was to understand whether the levels of inflammasomes were related to the severity of RA disease, which might provide a stronger theoretical basis for RA treatment.
Methods
The mRNA expression levels of some inflammasomes and associated molecules, including IL-1beta and IL-18, in peripheral blood mononuclear cells (PBMCs) from 30 RA patients (n = 30) and 16 healthy control (HC) individuals were determined by quantitative real-time polymerase chain reaction (qRT‒PCR), and the levels of plasma IL-1beta and IL-18 were also measured by enzyme-linked immunosorbent assay (ELISA). Moreover, the clinical characteristics and laboratory results of the patients were collected and analyzed in this study.
Results
The relative mRNA expression levels of NLRP3, NLRC4, AIM2, caspase-1, and IL-1beta were significantly higher and those of NLRP1, NLRP2 and NLRC5 were notably lower in the HC group than in the RA group. Moreover, the plasma IL-1beta and IL-18 levels were markedly increased in the RA group. Additionally, the mRNA level of AIM2 was negatively correlated with disease activity score 28 (DAS28) by stepwise linear regression analysis. erythrocyte sedimentation rate (ESR) was positively correlated with DAS28 by multiple linear regression analysis in the RA group.
Conclusions
These findings imply the critical role of NLRP3, NLRC4, AIM2, caspase-1 and plasma IL-1beta and IL-18 in the pathogenesis of RA patients, which provides potential targets for the treatment of RA.
Similar content being viewed by others
Background
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic erosive arthritis. The pathogenesis is marked by persistent, chronic inflammation of the synovial membrane. This leads to synovitis and damage to bone and joints, which ultimately causes joint deformity, loss of function, and even disability [1, 2]. The pathogenesis of RA remains unclear, although inflammasomes are considered to be closely involved in RA pathogenesis. Inflammasomes are an important component of the immune system. They identify pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), or homeostasis-altering molecular processes (HAMPs) on pathogens through pattern recognition receptors (PRRs), recognizing and eliminating exogenous pathogenic microorganisms or endogenous molecules released by the host itself [3, 4]. The canonical inflammasomes are composed of a sensor molecule, an adapter protein (apoptosis-associated speck-like protein containing a caspase recruitment domain, ASC), and an effector molecule [5]. The sensors include members of the NLR family, the absent in melanoma 2 (AIM2)-like receptor (ALR) family and pyrin, specifically NOD-like receptor protein 1 (NLRP1), NLRP2, NLRP3, NOD-like receptor family apoptosis inhibitory protein (NAIP), NLR family CARD domain-containing 4 (NLRC4), NLRC5, NLRP6, NLRP7, NLRP9, NLRP12, AIM2, human interferon (IFN) γ-inducible protein (IFI) 16 and pyrin [6, 7]. Caspases include human caspases-1/4/5 and mouse caspases-1/11 and active inflammatory caspases that cleave pro-IL-1beta and pro-IL-18 to produce active IL-1beta and IL-18. Thus, they exert proinflammatory effects and promote the cleavage of the pore-forming protein gasdermin D (GSDMD) to induce pyroptosis [5, 8].
Inflammasomes are expressed in immune cells, such as T cells, monocytes, and macrophages, and nonimmune cells, such as epithelial cells and myofibroblasts [5]. An appropriate inflammatory response is beneficial for the body. However, it will trigger excessive tissue damage and lead to disease when it is uncontrolled. While there are numerous reports on inflammasomes in RA, no systematic studies have been reported.
In this study, we assessed the mRNA levels of NLRP1, NLRP2, NLRP3, NLRC4, NLRC5, NLRP12, AIM2, CARD8, IFI16, Pyrin, NAIP, caspase-1, caspase-4, caspase-5, IL-1beta, and IL-18 in PBMCs from RA patients and HC individuals and conducted a pilot investigation of common inflammasomes and associated molecules to provide a stronger theoretical basis for RA treatment.
Materials and methods
Objective
This study aimed to perform a pilot study on the expression levels of inflammasomes and associated molecules to provide a stronger theoretical value for RA treatment.
Study design and participants
A total of 30 patients with RA were enrolled in this study; these patients included 10 males and 20 females. The average age of these patients was 55 years (range: 31 to 75 years). RA patients who fulfilled the revised American College of Rheumatology (ACR) 2010 criteria for RA were consecutively enrolled in the Shaoxing People’s Hospital between October 2021 and March 2022 [9]. RA patients with other inflammatory or autoimmune diseases, cancers, mental disorders or hormonal diseases and pregnant and lactating women were excluded from this study. Information on disease activity score 28 (DAS28), rheumatoid factor (RF), anti-cyclic citrullinated peptide antibodies (anti-CCP), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cell (WBC) count, neutrophil count, monocyte count, lymphocyte count and other data were collected. A total of 16 healthy control individuals (HC individuals) matched for sex and age were also enrolled. This group included 5 males and 11 females of 40 to 72 years of age who had no autoimmune or inflammatory diseases. The characteristics of these enrolled patients are summarized in Table 1.
Human PBMC separation and total RNA extraction
Fresh peripheral blood samples were collected into EDTA-K2-containing tubes and centrifuged at 800 × g for 5 min. Then, the plasma was collected into the tubes and stored at -80 °C. The remaining blood components were diluted with an equal volume of phosphate buffered saline (PBS), and then PBMCs were collected by gradient centrifugation according to the Lympholyte®-H (CEDARLANE, Netherlands) manufacturer’s protocols. The total RNA in PBMCs from each case was extracted using an RNA Extraction Kit (Vazyme Biotech, Nanjing, China) as quickly as possible according to the protocol. The integrity and concentration of the RNA were assessed by a NanoDrop 2000 (Thermo Scientific, USA) spectrophotometer.
qRT‒PCR analysis
Quantitative real-time polymerase chain reaction (qRT‒PCR) was carried out with the PrimeScript™ RT kit (Vazyme Biotech, Nanjing, China) and SYBR Premix Ex Taq™ II (Vazyme Biotech, Nanjing, China). The housekeeping gene encoding GAPDH was used as an internal control, and the primers are listed in Table 2. The relative expression levels of the target genes were analyzed using the 2−ΔΔCt method.
Enzyme-linked immunosorbent assay (ELISA)
The plasma concentrations of IL-1beta and IL-18 were determined by enzyme-linked immunosorbent assay (ELISA) according to the protocol of the kit (Boster Biological, Wuhan, China). The detection wavelength was 450 nm, and experiments were conducted in triplicate with consistent results.
Statistical analysis
Statistical analysis and graphic presentation were performed by SPSS version 25.0 and GraphPad Prism 9.0. Unpaired Student’s t test or one-way ANOVA was used when the data passed the normality test; otherwise, the nonparametric Mann–Whitney test or the Kruskal‒Wallis test was used. The correlation analysis was performed by multiple linear regression analysis and stepwise linear regression analysis, excluding the interference of confounding factors. Two-tailed P < 0.05 was considered to be statistically significant.
Results
Characteristics of study subjects
Thirty patients with RA and sixteen healthy adults were enrolled in this study. There was no sex or age differences between the RA group and the HC group. There was no significant difference in the counts of WBCs, monocytes and neutrophils between the two groups, but the number of lymphocytes in the RA group was higher than that in the HC group (P = 0.04).
The mRNA expression levels in the RA and HC groups
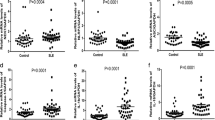
The mRNA expression levels of NLRP3, NLRC4, AIM2, caspase-1 and IL-1beta in the RA group were markedly increased (Fig. 1A-E), but the levels of NLRP1, NLRP2 and NLRC5 were notably decreased in comparison with those in the HC group (Fig. 1F-H). Moreover, there were no significant differences in NLRP12, CARD8, IFI16, pyrin, NAIP, Caspase-4/5 and IL-18 mRNA levels between the two groups (Supplementary material: Table S1). Although there was no significant difference in the relative expression levels of IL-18 mRNA between the two groups, the female group was markedly higher than the male group in RA patients (Fig. 1I).
Differential expression levels of inflammasomes and associated molecules. (A) The mRNA level of NLRP3. (B) The mRNA level of NLRC4. (C) The mRNA level of AIM2. (D) The mRNA level of caspase-1. (E) The mRNA level of IL-1beta. (F) The mRNA level of NLRP1. (G) The mRNA level of NLRP2. (H) The mRNA level of NLRC5. (I) The mRNA level of IL-18 in females with RA. RA, rheumatoid arthritis group; HC, healthy control group; M, male; F, female
Inflammasomes and DAS28-CRP in RA patients
To assess whether these inflammasomes and related proteins differed at different disease activity levels, the RA group was divided into high activity (DAS28-CRP > 5.1), moderate activity (3.2 < DAS28-CRP < 5.1), low activity (2.6 < DAS28-CRP < 3.2), and remission (DAS28-CRP < 2.6) groups according to DAS28-CRP [9]. The NLRP1 mRNA levels in the RA with different activity groups (low, moderate and high activity) were noticeably different from those in the HC group, and there were no significant differences between the RA remission group and the HC group. The relative expression levels of NLRP1 mRNA were not markedly different among the different RA activity groups (low, moderate and high activity) (Fig. 2A). The comparison between the active (including moderate and high activity groups) and inactive (low activity and remission groups) RA groups indicated that the mRNA levels of NLRP3 and caspase-1 in both the inactive and active groups were higher than those in the HC group, but there were no notable differences between the inactive and active groups (Fig. 2B, C). The levels of AIM2 mRNA in the inactive group were the highest and were significantly different from those in the HC group and the active group (Fig. 2D). The CRP and ESR levels in the high activity group were markedly higher than those in the low activity group and the remission group.
Differences in the expression of inflammasomes in different groups. (A) NLRP1 mRNA levels in the DAS28-CRP > 5.1 (high activity group), 3.2 < DAS28-CRP < 5.1 (moderate activity group), and 2.6 < DAS28-CRP < 3.2 (low activity group) groups were noticeably lower than those in the HC group, but there were no significant differences between the DAS28-CRP < 2.6 (remission group) and HC groups. (B) NLRP3 mRNA levels in the inactive and active groups were higher than those in the HC group. (C) Caspase-1 mRNA levels in the inactive and active groups were higher than those in the HC group. (D) AIM2 mRNA levels in the inactive group were higher than those in the HC group and the active group. IA, inactive group; A, active group
Measurement of plasma IL-1beta and IL-18
To determine whether there were differences in the plasma IL-1beta and IL-18 levels between RA patients and HC groups, these levels were determined by ELISA. The plasma IL-1beta (6.62 ± 1.65, P = 0.000) and IL-18 (759.79 ± 661.36, P = 0.001) levels in the RA group were noticeably higher than those in the HC group (Fig. 3A, B). There were noticeable significant differences among the active, inactive, and HC groups (Fig. 3C), but no difference was observed between the active and inactive groups. The level of IL-18 was the highest in the active group, which was markedly different from that in the HC group (Fig. 3D).
Differential expression assay of plasma IL-1beta and IL-18. (A), The plasma levels of IL-1beta in the RA group were noticeable higher than those in the HC group. (B) The plasma levels of IL-18 in the RA group were markedly higher than those in the HC group. (C) The plasma levels of IL-1beta in the inactive and active groups were higher than those in the HC group. (D) The plasma levels of IL-18 in the active group were higher than those in the HC group
Correlation between mRNA expression levels and clinical features of RA patients
To explore the relationships among the mRNA expression levels of these inflammasomes, plasma IL-1beta, IL-18 levels and clinical characteristics of RA patients, multiple linear regression analysis was used to analyze the relative mRNA expression levels of NLRP1, NLRP2, NLRP3, NLRC4, NLRC5, NLRP12, AIM2, CARD8, IFI16, Pyrin, NAIP, Caspase-1, Caspase-4, Caspase-5, IL-1beta, IL-18 mRNA and plasma IL-1beta and IL-18 levels and the clinical characteristics of RF, anti-CCP, CRP and ESR. However, no significant difference was found.
To find out whether the inflammasome mRNAs above affect plasma IL-1β and IL-18 levels, the results of stepwise regression analysis showed that the mRNA expression of inflammasome NLRP1 (β=-0.351, P = 0.017, 95% CI -3.068 to -0.323, R squared after adjustment 0.123) was negatively correlated with plasma IL-1β and that the mRNA expression of IL-18 (β=-0.351, P = 0.017, 95% CI -3.068 to -0.323, R squared after adjustment 0.123) was positively correlated with plasma IL-18 levels.
To understand the correlation between the expression level of these inflammasomes and related molecules and disease activity, multiple linear regression analysis and stepwise linear regression analysis were conducted. The results showed that the mRNA level of AIM2 was negatively correlated with DAS28 (β=-0.420, P = 0.021, 95% CI -1.832 to -0.162, R squared after adjustment 0.147). Additionally, to determine whether the laboratory findings were associated with DAS28, we compared the RF, anti-CCP, CRP, ESR, and neutrophil, monocyte and lymphocyte counts with DAS28 using multiple linear regression analysis. The results showed that ESR was positively correlated with DAS28 (β = 0.711, P = 0.01, 95% CI 0.013 to 0.083, R squared after adjustment 0.623).
Discussion
It is well known that the inflammasome is an important part of the innate immune system. Inflammasome activation triggers a series of inflammatory cascade responses, leading to the secretion of various cytokines, including the production of IL-1beta and IL-18. This has implications for the pathogenesis of many diseases, such as RA, systemic lupus erythematosus (SLE) and influenza virus infection [10]. In view of the role of inflammasomes in RA disease, we investigated the relative mRNA expression levels of common inflammasomes, including NLRP1, NLRP2, NLRP3, NLRC4, NLRC5, NLRP12, AIM2, CARD8, IFI16, Pyrin and related molecules, including NAIP, caspase-1, caspase-4, Caspase-5, IL-1beta and IL-18, in human PBMCs of RA patients and HC individuals.
The results showed that the relative mRNA expression levels of NLRP3, NLRC4, AIM2, caspase-1, and IL-1beta in the RA group were noticeably higher than those in the HC group. However, the relative mRNA expression levels of NLRP1, NLRP2, and NLRC5 were notably decreased in the RA group compared to those in the HC group. Interestingly, the mRNA levels of IL-18 were significantly higher in the female group than in the male group in RA patients. Although the lymphocyte count in the RA group was higher than that in the HC group, the two groups were comparable because the present study compared relative expression levels in human PBMCs. PBMCs mainly contain lymphocytes (including T-lymphocytes, B-lymphocytes, NK cells, etc.) and monocytes. In this study, inflammasomes mainly in monocytes and T lymphocytes of RA patients were activated, causing a cascade of reactions that affected the plasma levels of IL-1beta and IL-18 [10]. Therefore, we examined the plasma levels of IL-1beta and IL-18 in the RA and HC groups and found that the plasma IL-1beta and IL-18 levels in the RA group were markedly higher than those in the HC group. To further explore whether plasma IL-1beta and IL-18 can reflect disease activity and inflammation, we correlated inflammasome mRNA levels and cytokines with DAS2, CRP, ESR, RF and anti-CCP. The results showed that the mRNA level of AIM2 was negatively correlated with the DAS28 and that the ESR was positively correlated with the DAS28.
Wang et al. showed that NLRP3 and IL-1beta mRNA levels in human PBMCs were not significantly different between the RA patients and HC individuals, and caspase-1 mRNA levels were lower than controls. These results are different from the results of the present study, probably due to factors such as disease duration or drug use [11]. We divided NLRP3, caspase-1 and IL-1beta mRNA into five or three groups according to DAS28-CRP, and there was no noticeable difference between the groups (data not shown). These findings suggest that these mRNAs were not highly expressed due to increased disease activity. Some studies have shown that tofacitinib (TOF) attenuates NLRP3 activation and reduces serum IL-1beta production [12]. NLRP3 expression is increased in adjuvant arthritis (AA) and collagen-induced arthritis (CIA) animal models [13, 14]. Both IL-1beta and NLRP3 levels are increased in the PBMCs of RA patients [15, 16], suggesting that NLRP3 exacerbates the inflammatory response in RA. Moreover, inhibitors targeting NLRP3 have been applied to a variety of clinical diseases with good clinical efficacy [8]. Therefore, NLRP3 plays a pathogenic role in the disease process of RA.
Our study showed that AIM2 levels were significantly higher in the RA group than in the HC group, which was consistent with a previous report [17]. After we divided AIM2 into three groups according to disease activity, the results showed a significant difference between the active, inactive and HC groups. The highest AIM2 levels were found in the inactive group, which might explain why the relative mRNA levels of AIM2 were negatively correlated with DAS28. Animal models of AIM2 deficiency exhibit milder inflammatory responses and pathological changes [18, 19]. Although the AIM2 levels in RA patients were lower than those in HC individuals, fibroblast-like synovial cell (FLS) proliferation was inhibited after AIM2 was silenced in FLSs from RA patients [20]. Although the results were inconsistent, AIM2 remains a target for RA treatment according to the current findings. Additionally, there are fewer studies on NLRC4 in RA, and only one study from Brazil supports the role of NLRC4 in RA [21]. Our results showed higher NLRC4 levels in the RA group than in the HC group. In conclusion, both AIM2 and NLRC4 may play pathogenic roles in the RA disease process.
Gene polymorphisms of NLRP2 are associated with susceptibility to RA in the Chinese Han population [22]. There are no other reports on NLRP2 in RA, but the present study first found that the NLRP2 levels in PBMCs of RA patients were significantly lower than those in HC individuals. However, its function still needs to be further studied. In addition, we found that the mRNA levels of NLRP1 were also markedly lower in RA patients than in HC individuals, which is consistent with a previous report [11]. And NLRP1 mRNA level was negatively correlated with plasma IL-1beta. After the RA group was grouped by DAS28, there was no significant difference between these groups, indicating that NLRP1 was not associated with disease activity. Different results on whether genetic polymorphisms of NLRP1 are associated with RA have been reported, and some studies have also reported that inflammatory conditions in animal models can be attenuated by inhibiting the NLRP1 inflammasome [23,24,25]. In general, the role of NLRP1 in RA needs to be further explored with larger sample sizes that exclude the influence of other relevant factors. NLRC5 levels significantly increased in synovial tissues of AA rats and FLSs of RA patients, and inflammatory cytokines were significantly decreased after NLRC5 silencing [26,27,28].
Genetic polymorphisms of CARD8 have been most studied for their association with RA susceptibility or anti-TNF therapy [21, 29]. Caspase-4/5 is a downstream protein of the noncanonical pathway of the inflammasome that is rarely reported in RA, and genetic polymorphisms of caspase-5 are associated with an increased risk of RA development [30]. Studies have shown that NLRP12 negatively regulates the phosphorylation of signal transducer and activator of transcription 3 (STAT3), and the inflammatory response is exacerbated in the NLRP12−/− antigen-induced arthritis (AIA) mouse model [31, 32]. The roles of the inflammasomes mentioned above need to be further investigated.
The levels of plasma IL-1beta and IL-18 were significantly higher in RA patients than in HC individuals, and there was no significant difference between the groups when IL-1beta was divided into five or three groups. The use of leflunomide has been reported to decrease the serum level of IL-18 [33]. In this experiment, the study subjects were divided into a group using leflunomide, a group using hormones and a group using both drugs. The plasma level of IL-18 was analyzed between the three groups, and there was no significant difference in our study. Vasilev et al. reported that serum IL-18 levels were significantly reduced in females [34]. In this study, there was no significant difference in plasma IL-18 levels after grouping by sex, but IL-18 mRNA levels were notably lower in the female group than in the male group. Furthermore, IL-18 mRNA was correlated with plasma IL-18. The results of this study may also suggest the use of drugs that target IL-18 in the treatment of female patients with RA.
Conclusions
Although common inflammasomes have been gradually reported in RA, there are no systematic studies reporting multiple inflammasomes simultaneously. Our study is a more comprehensive report of common inflammasomes and their associated proteins in RA. Future studies with larger sample sizes in newly diagnosed patients with RA or with a single drug alone are needed due to sample size limitations and drug use. The human body is complex. The pathogenesis of diseases is not controlled by a single cytokine and involves complex interactions between proinflammatory and anti-inflammatory factors. For example, caspase-1 is a downstream protein of many inflammasomes, so its level can be increased or decreased by a variety of factors. Because of this, inhibitors targeting caspase-1 have also become the focus of research. For example, VX-740 (pralnacasan) attenuates osteoarthritis injury, and VX-765 (belnacasan) inhibits cytokine secretion in RA models [35, 36]. Additional inhibitors include MCC950, which directly targets the NACHT domain of the NLRP3 inflammasome, tranilast (TR) [37, 38], anakinra, which is an antagonist against the IL-1 receptor (IL-1Ra), and canakinumab, which is an anti-IL-1β monoclonal antibody [39, 40]. The latest study shows that NLRP3 gene polymorphisms elevated the susceptibility to RA disease, and have an impact on RF as well as anti-CCP titers in RA patients, and pyroptosis will also be a possible therapeutic target for RA [41, 42]. With the deepening of the research on the mechanisms of the inflammasome, inhibitors of the inflammasome itself or its related upstream and downstream proteins are gradually being applied.
RA is caused by a variety of factors, genetic or environmental, and its pathogenesis and therapeutic approaches are constantly evolving. Given the important role of inflammasomes in the disease process of RA, a deeper understanding of the mechanisms of action of each inflammasome is important for the development of RA treatment strategies. Overall, our findings indicate the important role of inflammasomes and associated molecules in the pathogenesis of RA patients and could contribute to the therapeutic strategy of RA.
Data Availability
All datasets generated for this study are included in the manuscript and the Supplementary material.
Abbreviations
- PBMCs:
-
Peripheral blood peripheral blood mononuclear cells
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- PAMPs:
-
Pathogen-associated molecular patterns
- DAMPs:
-
Damage-associated molecular patterns
- HAMPs:
-
Homeostasis-altering molecular processes
- PRRs:
-
Pattern recognition receptors
- NLRP3:
-
NOD-like receptor protein 3
- NLRC4:
-
NLR family CARD domain-containing 4
- AIM2:
-
Absent in melanoma 2
- IFI16:
-
Human interferon (IFN)g-inducible protein 16
- NAIP:
-
NOD-like receptor family apoptosis inhibitory protein
- GSDMD:
-
Pore-forming protein gasdermin D
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- RF:
-
Rheumatoid factor
- anti-CCP:
-
Anti-cyclic citrullinated peptide antibodies
- DAS28:
-
Disease activity score 28
References
Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–72. https://doi.org/10.1001/jama.2018.13103
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet (London England). 2016;388(10055):2023–38. https://doi.org/10.1016/s0140-6736(16)30173-8
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481(7381):278–86. https://doi.org/10.1038/nature10759
Kanneganti TD, Lamkanfi M, Núñez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27(4):549–59. https://doi.org/10.1016/j.immuni.2007.10.002
Xue Y, Enosi Tuipulotu D, Tan WH, Kay C, Man SM. Emerging activators and regulators of Inflammasomes and pyroptosis. Trends Immunol. 2019;40(11):1035–52. https://doi.org/10.1016/j.it.2019.09.005
Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–26. https://doi.org/10.1016/s1097-2765(02)00599-3
Chen L, Cao SQ, Lin ZM, He SJ, Zuo JP. NOD-like receptors in autoimmune diseases. Acta Pharmacol Sin. 2021;42(11):1742–56. https://doi.org/10.1038/s41401-020-00603-2
Jiang Q, Wang X, Huang E, Wang Q, Wen C, Yang G, et al. Inflammasome and its therapeutic targeting in rheumatoid arthritis. Front Immunol. 2021;12:816839. https://doi.org/10.3389/fimmu.2021.816839
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. https://doi.org/10.1002/art.27584
Shin JI, Lee KH, Joo YH, Lee JM, Jeon J, Jung HJ, et al. Inflammasomes and autoimmune and rheumatic diseases: a comprehensive review. J Autoimmun. 2019;103:102299. https://doi.org/10.1016/j.jaut.2019.06.010
Wang T, Zhu CL, Wang S, Mo LW, Yang GD, Hu J, et al. Role of NLRP3 and NLRP1 inflammasomes signaling pathways in pathogenesis of rheumatoid arthritis. Asian Pac J Trop Med. 2014;7(10):827–31. https://doi.org/10.1016/s1995-7645(14)60145-0
Yang X, Zhan N, Jin Y, Ling H, Xiao C, Xie Z, et al. Tofacitinib restores the balance of γδTreg/γδT17 cells in rheumatoid arthritis by inhibiting the NLRP3 inflammasome. Theranostics. 2021;11(3):1446–57. https://doi.org/10.7150/thno.47860
Guo C, Fu R, Wang S, Huang Y, Li X, Zhou M, et al. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 2018;194(2):231–43. https://doi.org/10.1111/cei.13167
Li XF, Shen WW, Sun YY, Li WX, Sun ZH, Liu YH, et al. MicroRNA-20a negatively regulates expression of NLRP3-inflammasome by targeting TXNIP in adjuvant-induced arthritis fibroblast-like synoviocytes. Joint bone Spine. 2016;83(6):695–700. https://doi.org/10.1016/j.jbspin.2015.10.007
Ruscitti P, Cipriani P, Di Benedetto P, Liakouli V, Berardicurti O, Carubbi F, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol. 2015;182(1):35–44. https://doi.org/10.1111/cei.12667
Choulaki C, Papadaki G, Repa A, Kampouraki E, Kambas K, Ritis K, et al. Enhanced activity of NLRP3 inflammasome in peripheral blood cells of patients with active rheumatoid arthritis. Arthritis Res Therapy. 2015;17(1):257. https://doi.org/10.1186/s13075-015-0775-2
Afroz S, Giddaluru J, Vishwakarma S, Naz S, Khan AA, Khan N. A Comprehensive Gene expression Meta-analysis identifies Novel Immune Signatures in Rheumatoid Arthritis Patients. Front Immunol. 2017;8:74. https://doi.org/10.3389/fimmu.2017.00074
Baum R, Sharma S, Carpenter S, Li QZ, Busto P, Fitzgerald KA et al. Cutting edge: AIM2 and endosomal TLRs differentially regulate arthritis and autoantibody production in DNase II-deficient mice. Journal of immunology (Baltimore, Md: 1950). (2015)194(3):873-7. https://doi.org/10.4049/jimmunol.1402573
Jakobs C, Perner S, Hornung V. AIM2 drives joint inflammation in a Self-DNA triggered Model of Chronic Polyarthritis. PLoS ONE. 2015;10(6):e0131702. https://doi.org/10.1371/journal.pone.0131702
Chen Y, Fujuan Q, Chen E, Yu B, Zuo F, Yuan Y, et al. Expression of AIM2 in rheumatoid arthritis and its role on Fibroblast-Like Synoviocytes. Mediat Inflamm. 2020;2020:1693730. https://doi.org/10.1155/2020/1693730
Addobbati C, da Cruz HLA, Adelino JE, Melo Tavares Ramos AL, Fragoso TS, Domingues A, et al. Polymorphisms and expression of inflammasome genes are associated with the development and severity of rheumatoid arthritis in brazilian patients. Inflamm Research: Official J Eur Histamine Res Soc [et al]. 2018;67(3):255–64. https://doi.org/10.1007/s00011-017-1119-2
Yang XL, Hu ZD, Wu Q, Liu X, Liu QJ, Zhang YC, et al. Association of polymorphisms in SPARC and NLRP2 genes with rheumatoid arthritis in a chinese Han population. Mod Rheumatol. 2015;25(1):67–71. https://doi.org/10.3109/14397595.2014.903595
Zhang L, Dong Y, Zou F, Wu M, Fan C, Ding Y. 11β-Hydroxysteroid dehydrogenase 1 inhibition attenuates collagen-induced arthritis. Int Immunopharmacol. 2013;17(3):489–94. https://doi.org/10.1016/j.intimp.2013.07.015
Goh LL, Yong MY, See WQ, Chee EYW, Lim PQ, Koh ET, et al. NLRP1, PTPN22 and PADI4 gene polymorphisms and rheumatoid arthritis in ACPA-positive singaporean chinese. Rheumatol Int. 2017;37(8):1295–302. https://doi.org/10.1007/s00296-017-3762-x
Sui J, Li H, Fang Y, Liu Y, Li M, Zhong B, et al. NLRP1 gene polymorphism influences gene transcription and is a risk factor for rheumatoid arthritis in han chinese. Arthritis Rheum. 2012;64(3):647–54. https://doi.org/10.1002/art.33370
Liu YR, Yang L, Xu QQ, Lu XY, Ma TT, Huang C, et al. Long noncoding RNA MEG3 regulates rheumatoid arthritis by targeting NLRC5. J Cell Physiol. 2019;234(8):14270–84. https://doi.org/10.1002/jcp.28126
Yu H, Ding C, Dai S, Sun J, Wang S, Zhang Z. Long noncoding RNA FER1L4 regulates rheumatoid arthritis via targeting NLRC5. Clin Exp Rheumatol. 2020;38(4):713–23.
Ji YR, Chen Y, Chen YN, Qiu GL, Wen JG, Zheng Y, et al. Dexmedetomidine inhibits the invasion, migration, and inflammation of rheumatoid arthritis fibroblast-like synoviocytes by reducing the expression of NLRC5. Int Immunopharmacol. 2020;82:106374. https://doi.org/10.1016/j.intimp.2020.106374
Sode J, Vogel U, Bank S, Andersen PS, Hetland ML, Locht H, et al. Genetic variations in pattern recognition receptor loci are Associated with Anti-TNF response in patients with rheumatoid arthritis. PLoS ONE. 2015;10(10):e0139781. https://doi.org/10.1371/journal.pone.0139781
Rui H, Yan T, Hu Z, Liu R, Wang L. The association between caspase-5 gene polymorphisms and rheumatoid arthritis in a Chinese population. Gene. (2018)642:307 – 12. https://doi.org/10.1016/j.gene.2017.11.032
Prado DS, Veras FP, Ferreira RG, Damasceno LEA, Melo PH, Zamboni DS, et al. NLRP12 controls arthritis severity by acting as a checkpoint inhibitor of Th17 cell differentiation. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2020;34(8):10907–19. https://doi.org/10.1096/fj.202000795R
Zhang X, Nan H, Guo J, Liu J. NLRP12 reduces proliferation and inflammation of rheumatoid arthritis fibroblast-like synoviocytes by regulating the NF-κB and MAPK pathways. Eur Cytokine Netw. 2021;32(2):15–22. https://doi.org/10.1684/ecn.2021.0465
Gualberto Cardoso PR, Diniz Lopes Marques C, de Melo Vilar K, Dantas AT, Branco Pinto Duarte AL, Pitta IDR et al. Interleukin-18 in Brazilian Rheumatoid Arthritis Patients: Can Leflunomide Reduce It? Autoimmune diseases. (2021)2021:6672987. https://doi.org/10.1155/2021/6672987
Vasilev G, Manolova I, Ivanova M, Stanilov I, Miteva L, Stanilova S. The role of IL-18 in addition to Th17 cytokines in rheumatoid arthritis development and treatment in women. Sci Rep. 2021;11(1):15391. https://doi.org/10.1038/s41598-021-94841-x
Wannamaker W, Davies R, Namchuk M, Pollard J, Ford P, Ku G, et al. (S)-1-((S)-2-{[1-(4-amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by inhibiting the release of IL-1beta and IL-18. J Pharmacol Exp Ther. 2007;321(2):509–16. https://doi.org/10.1124/jpet.106.111344
Rudolphi K, Gerwin N, Verzijl N, van der Kraan P, van den Berg W. Pralnacasan, an inhibitor of interleukin-1beta converting enzyme, reduces joint damage in two murine models of osteoarthritis. Osteoarthr Cartil. 2003;11(10):738–46. https://doi.org/10.1016/s1063-4584(03)00153-5
Coll RC, Hill JR, Day CJ, Zamoshnikova A, Boucher D, Massey NL, et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat Chem Biol. 2019;15(6):556–9. https://doi.org/10.1038/s41589-019-0277-7
Huang Y, Jiang H, Chen Y, Wang X, Yang Y, Tao J, et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol Med. 2018;10(4). https://doi.org/10.15252/emmm.201708689
Calabrese LH. Anakinra treatment of patients with rheumatoid arthritis. The Annals of Pharmacotherapy. 2002;36(7–8):1204–9. https://doi.org/10.1345/aph.1A396
Alten R, Gomez-Reino J, Durez P, Beaulieu A, Sebba A, Krammer G, et al. Efficacy and safety of the human anti-IL-1β monoclonal antibody canakinumab in rheumatoid arthritis: results of a 12-week, phase II, dose-finding study. BMC Musculoskelet Disord. 2011;12:153. https://doi.org/10.1186/1471-2474-12-153
Li RN, Ou TT, Lin CH, Lin YZ, Fang TJ, Chen YJ, et al. NLRP3 Gene Polymorphisms in Rheumatoid Arthritis and Primary Sjogren’s Syndrome Patients. Diagnostics (Basel Switzerland). 2023;13(2). https://doi.org/10.3390/diagnostics13020206
Wu D, Li Y, Xu R. Can pyroptosis be a new target in rheumatoid arthritis treatment? Front Immunol. 2023;14:1155606. https://doi.org/10.3389/fimmu.2023.1155606
Acknowledgements
We gratefully appreciate the native English speaking editors at American Journal Experts (AJE) for providing reputable English language editing service for our manuscript (Verification code: 7B81-52DF-6A31-4DF2-B3A5).
Funding
This work was supported by Zhejiang Medical Health Science and Technology Project (No. 2023RC105, 2022RC275), the Basic Public Welfare Research Plan of Zhejiang Province (LGD21H100001), Shaoxing People’s Hospital Youth Fund (No. 2022YA10), National Natural Science Foundation of China (No. 81871709), Suzhou Science and Technology Project (No. SYS2019109).
Author information
Authors and Affiliations
Contributions
DC, GY and RL conceived and designed the experiments. QJ, XW, XX, LH and GZ performed the experiments. QJ, XX and DC analyzed the data. QJ and XW wrote the manuscript. DC revised the manuscript, contributed reagents, materials. QJ and XW contributed equally to this work. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This project was approved by the Ethics Committee of the Shaoxing People’s Hospital (no. 2022-Y346-01) and all study protocols complied with the Declaration of Helsinki. All involved participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jiang, Q., Wang, X., Xu, X. et al. Inflammasomes in rheumatoid arthritis: a pilot study. BMC Rheumatol 7, 39 (2023). https://doi.org/10.1186/s41927-023-00353-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41927-023-00353-8