Abstract
N-terminal sequences play crucial roles in regulating expression, translation, activation and enzymatic properties of proteins. To reduce cell toxicity of intracellular trypsin and increase secretory expression, we developed a novel auto-catalyzed strategy to produce recombinant trypsin by engineering the N-terminus of mature Streptomyces griseus trypsin (SGT). The engineered N-terminal peptide of SGT was composed of the thioredoxin, glycine-serine linker, His6-tag and the partial bovine trypsinogen pro-peptide (DDDDK). Furthermore, we constructed a variant TLEI with insertion of the artificial peptide at N-terminus and site-directed mutagenesis of the autolysis residue R145. In fed-batch fermentation, the production of extracellular trypsin activity was significantly improved to 47.4 ± 1.2 U·ml−1 (amidase activity, 8532 ± 142.2 U·ml−1 BAEE activity) with a productivity of 0.49 U·ml−1·h−1, which was 329% greater than that of parent strain Pichia pastoris GS115-SGT. This work has significant potential to be scaled-up for microbial production of SGT. In addition, the N-terminal peptide engineering strategy can be extended to improve heterologous expression of other toxic enzymes.
Similar content being viewed by others
Introduction
Trypsin (EC 3.4.21.4), a serine protease, is used in leather bating, food processing, pharmaceuticals, clinical diagnoses and biochemical testing applications1,2. Currently, trypsin has been discovered in many mammalian and bacterial sources, and the trypsin extracted from bovine pancreas is a well-known commercial product. However, this conventional approach of producing trypsin from bovine pancreas cannot meet the high demand, and may be contaminated with pathogens or viruses3,4, which poses as a potential hazard to mammal immunogenicity when used in the pharmaceutical industry. Therefore, to circumvent these problems, trypsinogens originating from various organisms are heterologously expressed in different hosts. When trypsinogen is expressed in an Escherichia coli system, the majority of the over-produced protein is found in inclusion bodies5. Although bovine and shrimp trypsinogen are expressed in soluble form by Pichia pastoris, its activation is dependent on digestion with enterokinase6,7. As alternatives, the bovine trypsin gene is also studied in transgenic rice cells8 and Lactococcus lactis9; however, the production of active recombinant trypsin is low even after codon optimization. As a result, it is always a big challenge to efficiently produce active trypsin with microbial cell factories.
As a potential alternative candidate, Streptomyces griseus trypsin (SGT), which is composed of 223 residues with a molecular weight of 23.1 kDa, has 34.2% homology with bovine trypsin in amino acid sequence, including the active site residues (H57, D102 and S195), substrate binding region and substrate specificity10,11,12. Moreover, compared with bovine trypsin (BT), SGT possesses only three pair of disulfide bonds and higher catalytic efficiency (i.e., kcat/Km Table 1). As a result, the SGT encoding gene has been cloned and expressed in S. lividans13,14, S. griseus15 and Bacillus subtilis16. Recently, we realized its secretory and active expression in P. pastoris17. Furthermore, the results also confirmed previous hypothesis that activation of the SGT in S. griseus is not self-activated1. However, the detailed activation mechanism remains unresolved18. In comparison, bovine trypsinogen is activated by enterokinase cleavage at the P1 site (Lys) of the recognized pro-peptide VDDDDK (VD4K)19 or itself20,21,22,23,24. Although substitution of the native pro-peptide APNP with VD4K resulted in expression of SGT in inactive form, we found that the soluble hybrid trypsinogen could be activated following removal of VD4K by enterokinase in vitro, suggesting that the partial bovine trypsinogen pro-peptide VD4K could assist in expressing the SGT in trypsinogen form for avoiding intracellular toxicity to host17. By combining in silico simulations and the results in vitro experiments, several variants with different short pro-peptides have been recently constructed and used for improving SGT production in P. pastoris25. Similarly, several studies aimed at improving the production of target enzymes with engineered N-terminal regions have been reported in E. coli and P. pastoris26,27,28,29. Therefore, the combination of N-terminal engineering and other internal modification methods such as site-directed mutagenesis may represent an efficient and practical approach for improving the production and enzymatic properties of target enzymes.
In the present study, we have developed a novel self-activation strategy to decrease the protease toxicity to cells and improve the titer and enzymatic properties of SGT. Applying in silico simulations and in vitro experiments we rationally engineered the N-terminus of SGT with differently artificial peptides (Fig. 1). Enzyme activity and N-terminal sequencing results demonstrate that the rationally designed SGT variant achieved secretory expression with a self-activation pattern. In fed-batch fermentation (3 l fermenter), the titer of recombinant SGT was significantly enhanced from 14.4 U·ml−1 17 to 47.4 ± 1.2 U·ml−1 with 329% increase.
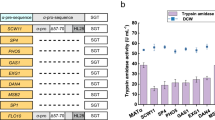
The artificial peptide segment of TLmt (D4K) is composed of TrxA (thioredoxin), GS*3 (GSGSGS, glycine-serine linker), 6H (His6-tag) and D4K (DDDDK, the partial bovine trypsinogen pro-peptide). In variants TLmt (D4R), TLmt (D4D) and TLmt (APNP), the pro-peptide D4K were substituted with DDDDR, DDDDD, DDDDD and APNP, respectively. Exmt represent a variant with the inserted peptide YVEF at N-terminus of SGT25.
Results and discussion
Effect of the pro-peptide D4K on SGT active expression
Streptomyces griseus trypsinogen consists of two segments: a pro-peptide and SGT. This structural architecture is similar to the domain arrangement of bovine trypsinogen (Supplementary Fig. S1). Nonetheless, the activation mechanism of SGT remains unresolved and is likely distinct from that of bovine trypsin, because the SGT was extracellularly expressed in inactive form after replacement of the SGT pro-peptide (APNP) with the partial bovine trypsinogen pro-peptide17. Currently, in vitro investigations combining in silico simulations with experiments are common approaches for studying the relationship between enzyme properties and structural changes30. Accordingly, the structure of the variant D4K-SGT with pro-peptide D4K at N-terminal was simulated to reveal the influence of the pro-peptide D4K on the conformation of SGT (PDB ID: 1SGT). The hybridized partial bovine trypsinogen pro-peptide D4K was found to be buried in D4K-SGT, which caused a loosened tertiary structure comparing with SGT (Fig. 2A). Moreover, the disulfide bonds (C42-C58, C168-C182, C191-C220) were mismatched in D4K-SGT (α. 2B) and an inaccurate catalytic triad conformation formed because of the changed hydrogen bonds (with disruption of the hydrogen bonds between D102 and H57) and substrate binding pocket (Fig. 2C), eventually resulting in an inactive state. The results also suggest that it would be attractive to release the bound peptide to give self-activation during the process of secretion, because overexpression of the active form of SGT is toxic to the host strain17.
Secretory production of active trypsin with a self-activation approach
To realize the auto-cleavage of the embedded artificial peptide and the activation of SGT, a number of N-terminal peptides were designed and fused to the N-terminus of SGT to generate SGT variants (Fig. 1). Specifically, TrxA (encoded by the trxA gene), which functions as a chaperone for assisting folding and accumulation of heterologous proteins31,32,33,34 was fused between the C-terminus of the α-factor signal peptide and the N-terminus of SGT. Moreover, a glycine-serine (GS*3) linker followed by a His6-tag (for facilitating purification of TrxA protein) was designed and inserted to avoid TrxA affecting the tertiary conformation of SGT. To achieve self-activation with elimination of the N-terminal peptides, the partial native bovine pro-peptide D4K (can be facilely recognized and cleaved by the recombinant SGT) and other pro-peptides were designed and comparatively investigated (Fig. 1). Accordingly, three dimensional structure of the variant TLmt (D4K) was simulated to evaluate our hypothesis. As shown in Fig. 3, the artificial peptide of TLmt (D4K) was outside of the mature SGT, which would be readily recognized and cleaved. In addition, no obvious alterations to the tertiary conformation of SGT (PDB ID: 1SGT) were observed, indicating active SGT could be released after autocatalysis.
To verify the above simulation results, all the recombinant strains containing the same copy numbers of variant rSGT genes were cultivated in flasks (Supplementary Fig. S2A). Notably, the variants TLmt (D4K) and TLmt (D4R) were successfully secreted in the active form. After induction for 144 h with 1% methanol (v/v), the trypsin enzyme activity (amidase) of TLmt (D4K) was 1.58 times higher than that of TLmt (D4R) (Fig. 4A), suggesting that the pro-peptide D4K was more readily recognized and catalyzed when compared with the D4R sequence. In contrast, no trypsin activity was detected in the cultures of the other variants, suggesting the catalytic specificity of SGT to amino acid residuals K or R.
(A) Trypsin amdidase activity of the variants with different pro-peptides, with 1% (v/v) methanol inducing for 144 h in flasks. (B) SDS-PAGE results of the culture supernatant of GS115-TLmt (D4K) variant (1) and GS115-SGT (2). (C) SDS-PAGE results of the purified active SGT from culture supernatant of GS115-TLmt (D4K) variant (1) and GS115-SGT (2). (D) SDS-PAGE result of the purified TrxA (3) from culture supernatant of GS115-TLmt (D4K) variant. Active SGT (Δ) and TrxA (▲). (E) The N-terminal five amino acids of the purified active SGT from TLmt (D4K).
To further verify the above results, the extracellular culture supernatant of the strain GS115-TLmt (D4K) was analyzed by SDS-PAGE with GS115-SGT as a control35. As shown in Fig. 4B–D, the bands corresponding to the active SGT (~ 28 kDa) and TrxA (~18 kDa) were clearly observed. Moreover, N-terminal sequencing results showed that the secreted active SGT shared the same initial five amino acids (VVGGT) (Fig. 4E) compared to the original sequence of 1SGT (PDB ID). Taken together, the results demonstrate that it is feasible and desirable to achieve self-activation of SGT by introducing artificial peptide.
Modification of the autolysis sensitive site R145 to improve the stability
In SGT, the autolysis loop (residues 141–152) has been reported to be flexible region and the primary target for autolysis36. In particular, the sensitive autolysis site R145 is recognized and cleaved by active trypsin37. Consequently, desensitization of the autolysis loops, especially the sensitive site, provide greater stability. As shown in Fig. 1, according to the simulation results (online sever http://dezyme.com/)38, six forward variants of Exmt (a variant of SGT with an inserted N-terminal peptide YVEF25) were constructed. The strains that contained the Exmt (R145 mutations) encoding gene with 9 copies (Supplementary Fig. S2B) were cultivated in flasks by inducing with 1% (v/v) methanol for 144 h. Then, the supernatant of each culture was purified for measuring specific activity to two substrates BAPNA and BAEE (Supplementary Fig. S3). Significantly, the mutant Exmt (R145I) showed the highest amidase specific activity (1,242.85 ± 99.15 U·mg−1) (Fig. 5A) and the highest esterase specific activity (101,491.58 ± 1,225.56 U·mg−1) (Supplementary Fig. S4), indicating the higher catalytic efficiency of the mutant Exmt (R145I). Subsequently, kinetic parameters of all the variants were further determined and compared. Although a decreased substrate affinity (i.e., Km toward BAPNA) was observed, the variant Exmt (R145I) showed the highest catalytic efficiency (i.e., kcat/Km) towards BAPNA (Table 1). The reason should be ascribed to the decreased Cα distance between S195 and H57 in catalytic triad (which benefits electron transportation)39,40 even with a narrowed distance between G216 and G226 in substrate binding pocket (Fig. 5C,D). Moreover, it could be found that Exmt (R145I) remained higher amidase activity than Exmt (Fig. 5B) after 15 days. In addition, both SDS-PAGE (Supplementary Fig. S5A,B) and molecular dynamics simulation RMSD (root mean square deviation) analysis results (Supplementary Fig. S6) also demonstrated that introduction of the mutation R145I improved the stability against self-autolysis.
Construction and fed-batch fermentation of the self-activation variants without autolysis
According to above results, N-terminal engineering gave the variant TLmt (D4K) secretory expression with a self-activation strategy that could be beneficial in reducing the protease toxicity of trypsin to the host strain. Simultaneously, site-directed mutagenesis of R145 to I significantly improved the stability and the catalytic efficiency. As a result, we further constructed the variants TLmt (R145I) and TLEI (Fig. 1), and comparatively investigated the trypsin activity with Exmt (R145I). As shown in Fig. 6, after 1% (v/v) methanol inducing for 144h, the amidase activity in the extracellular culture supernatant of the strains GS115-Exmt (R145I), GS115-TLmt (R145I) and GS115-TLEI were 4.97 ± 0.76, 6.53 ± 0.39 and 9.39 ± 0.36 U·ml−1, respectively, whereas no intracellular activity was detected.
To evaluate the trypsin production capacity of the strain GS115-TLEI, fed-batch fermentation with GS115-Exmt (R145I) as the control was further carried out. As shown in Fig. 7, the operation process was composed of three parts according to previous study41. Specifically, no trypsin was found to accumulate in the culture before induction. When methanol was added as the inducer, the amount of extracellular recombinant trypsin was rapidly accumulated. At 108 h, the amidase activity in supernatant of the GS115-Exmt (R145I) culture was increased to 18.4 ± 0.8 U·ml−1 (Fig. 7A). In comparison, the amidase activity in supernatant of the GS115-TLEI culture was accumulated to 47.4 ± 1.2 U·ml−1 (corresponding to 8532 ± 142.2 U·ml−1 esterase activity) at 96 h (Fig. 7B). Moreover, it could be found that compared with GS115-Exmt (R145I) the GS115-TLEI showed better cell growth and the biomass was accumulated to 130 g·l−1 (DCW) at 72 h, indicating the release of protease toxicity of the variant TLEI42. In addition, it was apparent that compared with GS115-Exmt (R145I), GS115-TLEI showed a lower decrease in the rate of the intracellular AOX activity. At 96 h, AOX activity of GS115-TLEI was present with a value of 68.37 ± 1.6 U·g–1 (Fig. 7D), which was 3.12-fold higher than that of GS115-Exmt (R145I) (Fig. 7C). Meanwhile, it could be found that no obvious intracellular amidase activity was detected in GS115-TLEI while the intracellular amidase activity of GS115-Exmt (R145I) was accumulated to 1.64 ± 0.13 U·ml−1 (Supplementary Fig. S7). The results suggested that it is desirable for improving cell activity and the secretory production of active trypsin by combining intramolecular modification and the self-activation strategy with N-terminal engineering. The mutant TLEI constructed here would have potential applications in production of trypsin at industrial scale.
In conclusion, although trypsin genes from different sources have been expressed in P. pastoris7,42,43 and the extracellularly expressed mature SGT was increased to 14.4 U·ml−1 (amidase activity) in 3-l fermenter22, the limits encountered are the protease toxicity of mature trypsin to the host cell and the instability because of self- autolysis. Therefore, development of a novel trypsin variant that can self-activate in secretory process is attractive. In the present study, inspired by the bovine trypsin activation mechanism and our previous results17, we constructed a number of variants and successfully achieved the secretory expression of trypsin with a self-activation strategy by fusion of an artificial peptide TrxA-GS*3-6H-D4K at the N-terminus of SGT. The site-directed mutagenesis of autolysis residue R145I was also engineered to improve stability against self-autolysis as well as catalytic efficiency. By combining these favorable factors, a self-catalyzed variant TLEI was constructed. In fed-batch fermentation, the production of the recombinant trypsin was significantly enhanced to 47.4 ± 1.2 U·ml−1 (amidase activity) with a productivity of 0.49 U·ml−1·h−1. In addition, the self-activation approach developed in this work could also be potentially used for production of other toxic enzymes.
Methods
Microorganisms and growth conditions
Streptomyces griseus ATCC 10137TM which produced the SGT was purchased from the American Type Culture Collection (ATCC, USA). P. pastoris GS115 (His−) and plasmid pPIC9K were both purchased from the InvitrogenTM (Beijing, China). The pMD19-T Sample vector was purchased from TAKARA (Dalian, China). Yeast nutrient medium, minimal dextrose medium, minimal medium, yeast extract peptone dextrose medium, buffered minimal glycerol-complex medium and buffered minimal methanol-complex medium were prepared by means of “P. pastoris expression Kit” (Pichia Multi-Copy Expression Kit, version A, Invitrogen BV, The Netherlands.).
Plasmid construction and yeast transformation
The recombinant plasmids were constructed with the single strand oligonucleotide (Supplementary Table S1). As showed in Fig. 1, The DNA fragment of TLmt variants encoding α-factor signal peptide, differently artificial peptide and mature SGT were constructed by overlap extension PCR with corresponding primers contained conserved sequence (Supplementary Table S1). And the resultant fragment were inserted into pPIC9K plasmid, digested with BamHI and NotI. Specially, the artificial peptide segment of TLmt (D4K) positioned between the C-terminus of the α-factor signal peptide and the N-terminus of SGT, which was composed of TrxA (thioredoxin, encoded by the trxA gene), GS*3 (GSGSGS, glycine-serine linker), 6H (His6-tag) and D4K (DDDDK, the partial bovine trypsinogen pro-peptide). In addition to the native bovine trypsinogen pro-peptide (D4K), other artificial peptides were also designed and comparatively studied (Fig. 1). The pPIC9K-Exmt (R145 mutations) and pPIC9K-TLEI were constructed by one step PCR according to Blunting Kination Ligation (BKL) Kit (TAKARA (TAKARA, Dalian, China)). pPIC9K-Exmt (R145 mutations) were amplified from previously constructed pPIC9K-Exmt plasmid with corresponding primers in (Supplementary Table S1), and pPIC9K-Tlmt (R145I) was constructed by primers Tlmt (R145I) 5 and Tlmt (R145I) 3 when pPIC9K-Tlmt (D4K) was used as temple. Then pPIC9K-TLEI was constructed from pPIC9K-Tlmt (R145I) with TLEI 5 and TLEI 3 primers. The constructed plasmids were transformed into P. pastoris GS115 (His−) by electroporation, according to P. Pastoris expression kit. The P. Pastoris strains harboring the variant SGT gene were screened in YPD plate with 4 mg·ml−1 geneticin.
Expression and purification of different SGT variants
The P. pastoris transformant was cultured in 250 ml baffled flask supplied with 25 ml buffered glycerol-complex medium at 30 °C at an agitation of 250 rpm for 24 h. Then the cells were centrifuged and resuspended in 30 ml of buffered methanol-complex medium to an OD600 value of 1.0, and shaken at 30 °C at an agitation of 250 rpm in 250 ml baffled flasks for 144 h. The culture broth was supplemented with 1% (v/v) methanol every 24 h to induce the expression of recombinant SGT. All of variants were purified with the same procedures. The crude sample was concentrated by precipitation with 25–55% ammonium sulfate. Then the precipitate was resuspended in 5 ml buffer A (10 mM Tris–HCl, pH 8.0, 10% (w/v) glycerol, and 1 mM EDTA) and dialyzed overnight against 100 ml of the same buffer. The sample was loaded onto a Hitrap benzamidine FF column (Φ1.6 × 2.5 cm, GE Healthcare), previously equilibrated with buffer A. Then, the column was washed with buffer B (10 mM NaOAc, pH 5.0, 10% glycerol, 1 mM EDTA, and 0.5 M NaCl). Proteins were eluted with 6 M guanidinium chloride solution at a flow rate of 2.5 ml·min−1, and fractions containing trypsin activity were collected and dialyzed against buffer A25. TrxA was purified by HisTrap FF crude 5 ml (GE Healthcare) according to HisTrap column instructions. Protein concentration was estimated by a Coomassie Brilliant Blue R-250 binding assay, using a commercial standard protein solution containing bovine G-globulin. Protein concentration of effluents was monitored at 280 nm.
SDS-PAGE analysis
The protein was fractionated by SDS-PAGE system. The samples (30 μl) were pretreated by mixing with 6 μl 5 × SDS-PAGE loading buffer in a 0.5 ml centrifugal tube. After vortexing for 5 seconds, the samples were incubated in 100 °C for 10 min, then each sample aliquot (10 μl) was loaded. Electrophoresis of the protein was performed on 12% separating gel with 5% stacking gel. The gels were kept at a constant voltage of 80 and 110 V at stacking and separating gel respectively, then stained with coomassie brilliant blue R-250. Sequencing five amino acids of N-terminal were done by Sangong Biotech Co., Ltd. (Shanghai, China).
Determination of trypsin activity
The cultures of different SGT variants, were collected every 24 h and diluted to the same cell concentration with assay buffer. Then supernatant was prepared for measuring activity, by centrifuging culture solution at 10000g and 4 °C for 5 min, with triplicates measured in an effort to reduce error. The pellets were washed twice with assay buffer and then resuspended and disrupted by high pressure homogenization (Constant Cell Disruption Systems). The cell-free extract was used for measuring intracellular activity. Trypsin amidase activity was determined by spectrophotometer as the release of p-nitroanilide by hydrolysis of the artificial substrate Nα-benzoyl-DL-arginine-p-nitroanilide (BAPNA)44. Briefly, sample (100 μl) was mixed with 800 μl of assay buffer (50 mM Tris-HCl, pH 8.0 at 37 °C) and 100 μl of 0.1 M BAPNA. The change in absorbance at 410 nm was monitored spectrophotometrically. One unit (U) of amidase was defined as the amount of enzyme required for producing a ΔA410 of 0. 1 per minute under the above conditions. The amidase activity of sample was calculated according to Equation 1:

where df is the dilution factor.
Trypsin esterase activity was measured using Nα-benzoyl-L-arginine ethyl ester hydrochloride (BAEE) as a substrate45. Immediately mix sample (200 μl) with 3 ml of assay buffer (67 mM, pH 7.6 sodium phosphate buffer containing 0.25 mM BAEE) at 25 °C. The change in absorbance at 253 nm was monitored spectrophotometrically. One unit (U) of esterase was defined as the amount of enzyme required for producing a ΔA253 of 0.001 per minute under the above conditions. The esterase activity of sample was calculated according to Equation 2:

where df is the dilution factor; 0.2 means that 0.2 ml of sample was added into the reaction system.
Molecular dynamics simulation and configuration of recombinant trypsin
The X-ray crystal structure of the wild type Streptomyces trypsin (PDB ID: 1SGT)11, downloaded from the RCSB Protein Data Bank (http://www.pdb.org/pdb/home/home.do), was used as the template for modeling. Stereo chemical analysis of the structure was performed using PROCHECK (http://nihserver.mbi.ucla.edu/SAVS/)46. The 3D model simulations were carried out by NAMD software with charm M force field (http://www.ks.uiuc.edu/Research/namd)47. Na+ was also added to neutralize the system. The pH 7.0 was set as default. Proteins were solvated in a cubic box consisting of TIP3P water molecules and the box size was chosen by the criterion that the distance of protein atoms was greater than 10.0 Å from the wall. A Ewald summation method was used for calculating the total electrostatic energy in a periodic box named Particle Mesh Ewald (PME). Structure minimization was performed to remove any unexpected coordinate collision and to get the local minima. The water box and the whole system were minimized using the descent method plus the conjugate gradient method48. After minimization, the system heating, equilibration and data sampling were carried out in turn. The system heating was performed gradually from 0 K to the desired temperature at a constant temperature and volume (NTV ensemble), followed by a further 150 ps simulation for equilibration and 3 ns or longer simulation for data sampling at constant temperature and constant pressure (NTP ensemble). The temperature was set as 300 K, 330 K respectively, at 1 atmospheric pressure. Structural diagrams were drawn using PyMol software (http://pym ol.sour ceforge.net/)49.
Illustration of the simulated models
The simulated models of the trypsin variants have structural parameters from primary sequence to tertiary structure. Among these parameters, the amino acids, H bonds, Ion pairs, π-interactions (secondary structure parameters), and secondary structure contents (tertiary structural parameter) are important for the investigation. The amino acids can be calculated by the Vector NTI software50; H bonds were predicted by PyMol software (http://pymol.sourceforge.net/)49.
Anti-autolysis comparison of SGT and recombinant SGT
Purified samples were diluted to same protein concentration (50 mg·l−1), then were incubated at 37 °C in dry bath for 15 days, with 1 ml enzyme solution in 1.5 ml eppendorf tube. The samples were collected to measure amidase activity every three days, initial activity defined as 100%. Triplicates were used to measure activity in an effort to reduce error.
Determination of copy number of recombinant SGT (rSGT) gene by real-time quantitative PCR
The genomic DNAs of strains were prepared for absolute quantification using the TIANamp Yeast DNA Kit (Tiangen Biotech, Beijing, China). GAPDH (gene ID 8198905 which presents on the chromosome 2 of P. pastoris) was chosen as the reference gene. The pPIC9K-rSGT and pMD19-T-GAPDH were constructed to establish standard curves, and the amount of plasmid was measured using a Qubit® 2.0 fluorometer (Life Technologies, USA). Real-time PCR primers were designed using Beacon Designer 7 software (Supplementary Table S1). The PCR mixture were prepared using SYBR® Premix Ex TaqTM II (Tli RNaseH Plus) (TAKARA, Dalian, China), and amplification was performed using a Light Cycler® 480 instrument (Roche Diagnostics Ltd., Rotkreuz, Switzerland). For each sample, reactions were performed in triplicates. Standard curves were generated by a linear regression of the mean Ct values plotted against log10 of diluted template. The copy number of the target gene was determined according to Equation 351.

Production of recombinant trypsin by fed-batch fermentation
After cultivating the cells at 30 °C and 200 rpm for 24 h in a 500 ml shake flask containing 50 ml YPD (20 g·l−1 glucose, 20 g·l−1 peptone, 10 g·l−1 yeast extract), 10% (v/v) of the inoculum was inoculated into a 3 l fermenter (LiFlus GM BioTRON, Korea) with 800 ml basal salts medium (BSM: 40 g·l−1 glycerol, 18 g·l−1 K2SO4, 4.13 g·l−1 KOH, 14.9 g·l−1 MgSO4·7H2O, 27 ml·l−1 H3PO4, 0.93 g·l−1 CaSO4) with 4.4 ml·l−1 PTM1 trace salts (PTM1: 6 g·l−1 CuSO4·5H2O, 0.09 g·l−1 KI, 3 g·l−1 MnSO4·H2O, 0.02 g·l−1 H3BO3, 0.2 g·l−1 MoNa2O4·2H2O, 0.5 g·l−1 CoCl2, 20 g·l−1 ZnCl2, 65 g·l−1 FeSO4·7H2O, 0.2 g·l−1 biotin, 5.0 ml·l−1 H2SO4). The pH of the medium was controlled at 5.5 with the addition of 50% ammonium hydroxide and 30% phosphoric acid. The temperature was controlled at 30 °C, and dissolved oxygen (DO) level was maintained over 30% of air saturation by a cascaded control of agitation rate (500–1000 rpm) and aeration rate (3–5 l·min−1). The fermentation process was composed of three phases. The fermentation began with glycerol batch phase until the initial glycerol was exhausted, as indication of DO level increased to more than 50%. In glycerol fed-batch phase, the feeding medium was pumped into the fermenter according to a predetermined protocol, which contained 50% (w/v) glycerol and 12 ml·l−1 PTM1 solution. Finally, the trypsin was expressed with induced by methanol in methanol fed-batch phase. Two hours after depletion of the glycerol with DO increased to more than 50% and dry cell weight (DCW) reached to 80 g·l−1, 1.8% (v/v) methanol containing 12 ml·l−1 PTM1 solution was fed with pH controlled at 5.5 and temperature controlled at 30 °C. The methanol concentration was maintained by the methanol on-line control station (FC2002, East China University of Science and Technology)52.
Determination of cell concentration, residual glycerol, biomass and intracellular alcohol oxidase activity
Cell concentration was measured spectrophotometrically at 600 nm with the appropriate dilution. 10 ml sample was collected every 12 h, then centrifuged in a weighed centrifuge tube at 10,000 g and 4 °C for 10 min. The supernatant was prepared to detect the residual glycerol by HPLC with refractive index detector. The tubes containing the pellets were dried to constant weight at 80 °C for measuring DCW. Triplicates were used to determining activity in an effort to reduce error.
For intracellular alcohol oxidase (AOX) activity analysis, 1 ml sample was centrifuged under the above conditions. The pellets were washed twice with 50 mM phosphate buffer (pH 7.0), then resuspended in the same buffer and disrupted by high pressure homogenization (Constant Cell Disruption Systems). The cell-free extract was prepared to measure AOX activity by collecting centrifuged supernatant. Triplicates were used to determining activity in an effort to reduce error.
The intracellular AOX activity was assayed by measuring H2O2 produced during oxidation of methanol. The standard assay mixture contained 100 μM phosphate buffer (pH 7.0), 4.3 μM phenol, 15 IU peroxidase, 1 μM 4-aminoantipyrine, 400 μM methanol, and cell-free extract in a total volume of 3 ml. The reaction was carried out at 37 °C for 10 min, then the increase in absorbance at 500 nm was measured. One enzyme unit is defined as production of one μmol of H2O2 per minute53.
Additional Information
How to cite this article: Zhang, Y. et al. Improved Production of Active Streptomyces griseus Trypsin with a Novel Auto-Catalyzed Strategy. Sci. Rep. 6, 23158; doi: 10.1038/srep23158 (2016).
References
Olafson, R. W., Jurasek, L., Carpenter, M. R. & Smillie, L. B. Amino acid sequence of Streptomyces griseus trypsin. Cyanogen bromide fragments and complete sequence. Biochemistry 14, 1168–1177 (1975).
Trop, M. & Birk, Y. The specificity of proteinases from Streptomyces griseus (pronase). Biochem J 116, 19–25 (1970).
Lombardi, J., Woitovich Valetti, N., Picó, G. & Boeris, V. Obtainment of a highly concentrated pancreatic serine proteases extract from bovine pancreas by precipitation with polyacrylate. Sep Purif Technol 116, 170–174 (2013).
Malpiedi, L. P., Romanini, D., Picó, G. A. & Nerli, B. B. Purification of trypsinogen from bovine pancreas by combining aqueous two-phase partitioning and precipitation with charged flexible chain polymers. Sep Purif Technol 65, 40–45 (2009).
Zhao, M., Wu, F. & Xu, P. Development of a rapid high-efficiency scalable process for acetylated Sus scrofa cationic trypsin production from Escherichia coli inclusion bodies. Protein Expr Purif 116, 120–126 (2015).
Guerrero-Olazaran, M., Escamilla-Trevino, L. L., Castillo-Galvan, M., Gallegos-Lopez, J. A. & Viader-Salvado, J. M. Recombinant shrimp (Litopenaeus vannamei) trypsinogen production in Pichia pastoris . Biotechnol Prog 25, 1310–1316 (2009).
Hanquier, J. et al. A single mutation in the activation site of bovine trypsinogen enhances its accumulation in the fermentation broth of the yeast Pichia pastoris . Appl Environ Microbiol 69, 1108–1113 (2003).
Kim, N.-S. et al. Production of functional recombinant bovine trypsin in transgenic rice cell suspension cultures. Protein Expr Purif 76, 121–126 (2011).
Yao, L. et al. Expression of bovine trypsin in Lactococcus lactis . Int Dairy J 20, 806–809 (2010).
Olafson, R. W. & Smillie, L. B. Enzymic and physicochemical properties of Streptomyces griseus trypsin. Biochemistry 14, 1161–1167 (1975).
Read, R. J. & James, M. N. Refined crystal structure of Streptomyces griseus trypsin at 1.7 Å resolution. J Mol Biol 200, 523–551 (1988).
Sidhu, S. S., Kalmar, G. B., Willis, L. G. & Borgford, T. J. Protease Evolution in Streptomyces griseus discovery of a novel dimeric enzyme J Biol Chem 270, 7594–7600 (1995).
Koo, B.-J., KWANG, H. B., Byun, S.-M. & Hong, S.-K. Purification and characterization of Streptomyces griseus trypsin overexpressed in Streptomyces lividans . J Microbiol Biotechnol 8, 333–340 (1998).
Koo, B.-J., Kim, J.-M., Byun, S.-M. & Hong, S.-K. Optimal production conditions of Streptomyces griseus trypsin (SGT) in Streptomyces lividans . BMB Reports 32, 86–91 (1999).
Oh, E. A., Kim, M. S., Chi, W. J., Kim, J. H. & Hong, S. K. Characterization of the sgtR1 and sgtR2 genes and their role in regulating expression of the sprT gene encoding Streptomyces griseus trypsin. FEMS Microbiol Lett 276, 75–82 (2007).
Page, M. J., Wong, S.-L., Hewitt, J., Strynadka, N. C. & MacGillivray, R. T. Engineering the primary substrate specificity of Streptomyces griseus trypsin . Biochemistry 42, 9060–9066 (2003).
Ling, Z. et al. Functional expression of trypsin from Streptomyces griseus by Pichia pastoris . J. Ind Microbiol Biotechnol 39, 1651–1662 (2012).
Kim, J. C., Cha, S. H., Jeong, S. T., Oh, S. K. & Byun, S. M. Molecular cloning and nucleotide sequence of Streptomyces griseus trypsin gene. Biochem Biophys Res Commun 181, 707–713 (1991).
Chao, L. & Liener, I. E. Autoactivation of porcine trypsinogen in the presence and absence of calcium. Biochimica et biophysica acta 96, 508–516 (1965).
Carter, P. & Wells, J. A. Dissecting the catalytic triad of a serine protease. Nature 332, 564–568 (1988).
Kay, J. & Kassell, B. The autoactivation of trypsinogen. J Biol Chem 246, 6661–6665 (1971).
Ohta, Y. et al. Pro-peptide as an intermolecular chaperone: renaturation of denatured subtilisin E with a synthetic pro-peptide. Mol Microbiol 5, 1507–1510 (1991).
Chen, J. M. et al. Evolution of trypsinogen activation peptides. Mol Biol Evol 20, 1767–1777 (2003).
Nemoda, Z. & Sahin-Tóth, M. Human cationic trypsinogen. The tetra-aspartate motif in the activation peptide is essential for autoactivation control, but not for enteropeptidase recognition. J Biol Chem 280, 29645–29652 (2005).
Ling, Z. et al. Improvement of catalytic efficiency and thermostability of recombinant Streptomyces griseus trypsin by introducing artificial peptide. World J Microbiol Biotechnol 30, 1819–1827 (2014).
Cheong, D. E. et al. Enhancing functional expression of heterologous proteins through random substitution of genetic codes in the 5′ coding region. Biotechnol Bioeng 112, 822–826 (2014).
Goodman, D. B., Church, G. M. & Kosuri, S. Causes and effects of N-terminal codon bias in bacterial genes. Science 342, 475–479 (2013).
Gu, C., Zheng, F., Long, L., Wang, J. & Ding, S. Engineering the expression and characterization of two novel laccase isoenzymes from Coprinus comatus in Pichia pastoris by fusing an additional ten amino acids tag at N-terminus. PloS one 9, e93912 (2014).
Kim, S. K. et al. Simple amino acid tags improve both expression and secretion of Candida antarctica lipase B in recombinant Escherichia coli . Biotechnol Bioeng 112, 346–355 (2014).
R. Mudra, D., E Desino, K. & V Desai, P. In silico, in vitro and in situ models to assess interplay between CYP3A and P-gp. Curr Drug Metab 12, 750–773 (2011).
LaVallie, E. et al. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the Escherichia coli cytoplasm. Nat Biotechnol 11, 187–193 (1993).
Jonsdottir, G., Bjarnason, J. B. & Gudmundsdottir, A. Recombinant cold-adapted trypsin I from Atlantic cod-expression, purification, and identification. Protein Expr Purif 33, 110–122 (2004).
Palsdottir, H. M. & Gudmundsdottir, A. Expression and purification of a cold-adapted group III trypsin in Escherichia coli . Protein Expr. Purif. 51, 243–252 (2007).
Berndt, C., Lillig, C. H. & Holmgren, A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim Biophys Acta 1783, 641–650 (2008).
Ling, Z. et al. Rational design of a novel propeptide for improving active production of Streptomyces griseus trypsin in Pichia pastoris . Appl Environ Microbiol 79, 3851–3855 (2013).
Lee, W. S., Park, C. H. & Byun, S. M. Streptomyces griseus trypsin is stabilized against autolysis by the cooperation of a salt bridge and cation-π interaction. J Biochem 135, 93–99 (2004).
Bier, M., Nord, F. F. & Terminiello, L. On the mechanism of enzyme action. LXI. The self digestion of trypsin, calcium-trypsin and acetyltrypsin. Arch Biochem Biophys 65, 120–131 (1956).
Gonnelli, G., Rooman, M. & Dehouck, Y. Structure-based mutant stability predictions on proteins of unknown structure. J Biotechnol 161, 287–293 (2012).
Weiner, S., Seibel, G. & Kollman, P. The nature of enzyme catalysis in trypsin. Proc Natl.Acad Sci USA 83, 649–653 (1986).
Kraut, J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem 46, 331–358 (1977).
Siegel, R. & Brierley, R. Methylotrophic yeast Pichia pastoris produced in high-cell-density fermentations with high cell yields as vehicle for recombinant protein production. Biotechnol Bioeng 34, 403–404 (1989).
Viader-Salvado, J. M. et al. Shrimp (Litopenaeus vannamei) trypsinogen production in Pichia pastoris bioreactor cultures. Biotechnol Prog 29, 11–16 (2013).
Paulova, L., Hyka, P., Branska, B., Melzoch, K. & Kovar, K. Use of a mixture of glucose and methanol as substrates for the production of recombinant trypsinogen in continuous cultures with Pichia pastoris Mut+ . J Biotechnol 157, 180–188 (2012).
Chi, W. J. et al. Medium optimization and application of affinity column chromatography for trypsin production from recombinant Streptomyces griseus . J Microbiol Biotechnol 19, 1191–1196 (2009).
Erlanger, B. F., Kokowsky, N. & Cohen, W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys 95, 271–278 (1961).
Laskowski, R. A., Rullmann, J. A. C., MacArthur, M. W., Kaptein, R. & Thornton, J. M. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR 8, 477–486 (1996).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J Comput Chem 26, 1781–1802 (2005).
Jiang, W. et al. High-performance scalable molecular dynamics simulations of a parizable force field based on classical drude oscillators in NAMD. J Phys Chem Lett 2, 87–92 (2011).
DeLano, W. L. The PyMOL molecular graphics system. DeLano Scientific: San Carlos, CA, USA. (2002).
Lu, G. & Moriyama, E. N. Vector NTI, a balanced all-in-one sequence analysis suite. Brief Bioinform 5, 378–388 (2004).
Abad, S. et al. Real-time PCR-based determination of gene copy numbers in Pichia pastoris . Biotechnol J 5, 413–420 (2010).
Wang, Z. et al. Enhancement of cell viability and alkaline polygalacturonate lyase production by sorbitol co-feeding with methanol in Pichia pastoris fermentation. Bioresour Technol 101, 1318–1323 (2010).
Suye, S.-i., Ogawa, A., Yokoyama, S. & Obayashi, A. Screening and identification of Candida methanosorbosa as alcohol oxidase-producing methanol using yeast. Agric Biol Chem 54, 1297–1298 (1990).
Acknowledgements
This work was financially supported by the Natural Science Foundation of Jiangsu Province (BK20141107), a grant from the Key Technologies R & D Program of Jiangsu Province, China (BE2014607); Program for Changjiang Scholars and Innovative Research Team in University (No. IRT_15R26); China Postdoctoral Science Foundation funded project (125960) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
Y.Z. and Z.L. contributed equally to this work. Z.K. conceived the idea and designed research; Y.Z. and Z.L. performed the experiments; Z.K., Y.Z. and Z.L. analyzed the data and wrote the paper; G.D. and J.C. contributed to revise the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Zhang, Y., Ling, Z., Du, G. et al. Improved Production of Active Streptomyces griseus Trypsin with a Novel Auto-Catalyzed Strategy. Sci Rep 6, 23158 (2016). https://doi.org/10.1038/srep23158
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep23158
- Springer Nature Limited
This article is cited by
-
Enhanced catalytic efficiency and substrate specificity of Streptomyces griseus trypsin by evolution-guided mutagenesis
Systems Microbiology and Biomanufacturing (2023)
-
Improving production of Streptomyces griseus trypsin for enzymatic processing of insulin precursor
Microbial Cell Factories (2020)
-
High-level constitutive expression of leech hyaluronidase with combined strategies in recombinant Pichia pastoris
Applied Microbiology and Biotechnology (2020)
-
Recent advances of molecular toolbox construction expand Pichia pastoris in synthetic biology applications
World Journal of Microbiology and Biotechnology (2017)