Abstract
In most species of social insect the queen signals her presence to her workers via pheromones. Worker responses to queen pheromones include retinue formation around the queen, inhibition of queen cell production and suppression of worker ovary activation. Here we show that the queen signal of the Brazilian stingless bee Friesella schrottkyi is a mixture of cuticular hydrocarbons. Stingless bees are therefore similar to ants, wasps and bumble bees, but differ from honey bees in which the queen's signal mostly comprises volatile compounds originating from the mandibular glands. This shows that cuticular hydrocarbons have independently evolved as the queen's signal across multiple taxa and that the honey bees are exceptional. We also report the distribution of four active queen-signal compounds by Matrix-assisted laser desorption/ionization (MALDI) imaging. The results indicate a relationship between the behavior of workers towards the queen and the likely site of secretion of the queen's pheromones.
Similar content being viewed by others
Introduction
Queens of social insects indicate their presence in the colony through chemical signals1,2. Queen signals mediate a variety of queen-worker interactions including worker retinue formation around the queen, queen feeding thorough trophallaxis, inhibition of queen cell construction and suppression of worker reproduction among others1,2,3,4,5. The best-studied example of queen chemical communication is the queen mandibular pheromone (QMP) of the honey bee, Apis mellifera, which comprises more than nine compounds3. In contrast to the honey bees, ant, wasp and bumble bee queens use cuticular hydrocarbons and esters to signal their presence in the nest2,5.
Stingless bees are a diverse tribe of eusocial bees of mainly tropical distribution6. They are phylogenetically closest to the bumble bees7, but because the queens are strongly distinct from workers and because colonies are long-lived rather than established annually by independent queens, the biology of stingless bees is more similar to that of honey bees than bumble bees8.
Stingless bees live in colonies comprising hundreds or thousands of workers and a single queen. Only queens can lay fertilised, female-destined eggs, but workers retain ovaries in most genera and some workers lay unfertilised eggs that are either eaten by the queen as a food source or develop into males by parthenogenesis9. Despite the ecological importance of the stingless bees, the means by which queens regulate worker fertility is currently unknown. Identifying the queen pheromone of stingless bees would show if they are more honey bee-like or ant- and wasp-like and could indicate whether honey bees are unique in their primary system of queen-worker signalling.
Similarly to the honey bees, workers of the stingless bee Friesella schrottkyi remain sterile in a queen's presence, but they activate their ovaries and lay eggs in queenless colonies10. Chemical analysis of the mandibular secretions of F. schrottkyi workers and queens showed no evidence of a queen pheromone comparable to the QMP of honey bees11. On the other hand, cuticular extracts of workers differ substantially from those of a queen11, suggesting that cuticular hydrocarbons may act as signals of queen presence and regulate worker ovary activation.
In stingless bees queen oviposition is accompanied by highly ritualised interactions between queens and workers12. First, all the food necessary for development from egg to adult is regurgitated by workers into a newly-constructed brood cell. Second, following cell provisioning, the queen lays an egg on the top of the food. Finally, the workers seal the cell permanently13,14. The hatched larva feeds on the available food, pupates and emerges from the cell as an adult15. The ritualized interaction between workers and queen during the oviposition phase is characterised by intense antennation of the queens' head and first pair of legs by the workers, suggesting the presence of chemical signals specific to these areas of the queen's body13,14.
The abdomen of stingless bee queens has several glands that secrete their products onto the cuticle16. Integument glands are present on both the dorsal and ventral surfaces of the abdomen and the Dufour's gland has it's egress at the tip of the abdomen16,17. The function of the Dufour's gland is unclear, but in some bumble bees the contents of the Dufour's gland matches that of the cuticular profile18. In stingless bees, the Dufour's gland is much more developed in queens than it is in workers, suggesting that cuticular hydrocarbons secreted by the Dufour's gland may provide the queen signal in stingless bees.
Most studies of insect cuticular hydrocarbons have relied on gas chromatography and electron ionization mass spectrometry19,20. These techniques provide information about the presence of compounds in particular tissues, but do not provide information on the spatial distribution of compounds, the source of compounds or their effects on the behaviour of workers21,22,23. Recently, a laser-based technology, Imaging Mass Spectrometry (IMS), has been developed, which provides researchers with a means to identify the specific location of compounds in two dimensional space23,24,25. For example, Vrkoslav et al23 applied MALDI imaging to determine the spatial distribution of neutral lipids on the wings of flies (Neobellieria bullata and Drosophila melanogaster). However, thus far application of MALDI imaging has been restricted to planar surfaces such as insect wings.
Here we determine whether, the cuticular hydrocarbons of the F. schrottkyi queen act as a queen signal that modulates the activation of worker ovaries. First we show that extracts of long-chain hydrocarbons from the cuticular surface of queens are sufficient to reduce ovary activation in workers. Second, we demonstrate that ten queen-specific hydrocarbons cause neuronal firing in the antennae of workers. Finally we show that the spatial distribution of hydrocarbons over the cuticular surface of the queen supports their biological function as a queen signal.
Results
Suppression of worker ovary activation by extracts of queen cuticle
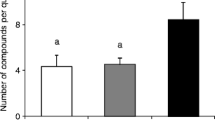
Significantly (Binomial GLMM: z = 3.43, p <0.001, n = 14 colonies) fewer workers from control queeright colonies and colonies treated with queen extracts (z = 2.31, p = 0.02, n = 14 colonies) had activated ovaries relative to workers sampled from queenless colonies (Fig. 1). The number of workers with activated ovaries did not differ between the control queenright group and the queen extract group (z = 1.42, p = 0.15, n = 14 colonies). Thus hexane extracts of whole bodies of F. schrottkyi queens were sufficient to reduce the frequency of worker ovary activation in queenless colonies to that seen in control queenright colonies even after 10 days (Fig 1.)
Ovary activation of F. schrottkyi workers under different treatments.
(A), worker with inactivated ovaries, (B) detail of the inactivated ovary (C) worker with activated ovaries, (D) details of activated worker ovary and (E) Levels of ovary activation under different treatment: Control, queenright colonies; Queenless, queenless colonies treated with pure hexane; Extract, queenless colonies treated with queen cuticular extract.
Electroantenography test
From the 37 hydrocarbons identified on the cuticle of F. schrottkyi queens11, ten produced an electrical response in worker antennae (Table S1 - Supplementary Information). The active compounds were the linear alkanes C21, C23, C24 and C25 and the methyl-branched alkanes 11-MeC21, 11-MeC23 and 11-MeC25 (Fig. S1 - Supplementary Information). Tests with synthetic compounds of the same structure produced similar electrical responses in antennae to those produced by the natural compounds from the queen extracts (Fig. S2 - Supplementary Information).
MALDI-MS analyses of standards
The synthetized and commercial standards were analysed by MALDI-MS to confirm and select the best methods for their ionizations and to understand the influence of the ions from the lithium 2,5-dihydroxybenzoate (LiDHB) matrix. All the evaluated compounds were successfully ionized, but because some ions from the matrix showed m/z ratios near to the compounds present on the samples, as observed in the spectra, the use of a high resolution analyser was essential for reliable data collection (Fig. S4–S7, Supplementary Information).
Comparison between head and body cuticular profiles of queens by GC-MS
The GC-MS analyses of isolated queen heads showed that the most abundant hydrocarbon on the head is the C21, which constitutes over 20% of the head's profile (Table S2 – Supplementary Information). In contrast, the results for the entire body show that the linear C25 is the most abundant compound and contributes over 40% of the queen's cuticular profile (Table S2 – Supplementary Information).
Spatial distribution of cuticular compounds by MALDI imaging
The head and metasomal parts of a queen were dissected and analysed by MALDI imaging. The ions m/z 303, 317, 345 and 359 [M+7Li]+ are related to C21, C22, C24 and C25 and it was possible to confirm from GC-MS data that they correspond to the linear C21, methyl branched C21, methyl branched C23/linear C24 and linear C25 compounds respectively. The distribution of these compounds over the head of the queen were evaluated through MALDI imaging(Fig. 2). Notably the ions at m/z 303, 317, 345 and 359 showed higher intensities in the frons area at the front of the head (Fig. 2A,C,D,E,H). The main ion (m/z 303 [M+7Li]+) on the head is the linear C21 (Table S2, Fig. 2A). A comparison between the dorsal and ventral metasomas, corresponding to terga T2-T7 and sterna S2-S7 respectively, showed higher ion intensities of m/z 303, 317 and 345 (C21, 11-MeC21 and C24, respectively) on the dorsal (tergal) metasaoma than the ventral (sternal). Higher intensities of ions m/z 303, 317 and 345 were observed in terga T7 and sterna (S7), while for m/z 359 (C25) the highest intensity was on terga T4 (Fig. 3).
MALDI-MS images reconstructed with ions m/z 303.36 [M+7Li]+ (C21) (A – front head, B- back head), 317.38 [M+7Li]+ (C22) (C – front head, D- back head), 345.41 [M+7Li]+ (C24) (E – front head, F- back head) and 359.42 [M+7Li]+ (C25) (G – front head, H- back head). Optical images of dissected front (I) and back head (J) of queen.
MALDI-MS images reconstructed with ions m/z 303.36 [M+7Li]+ (C21) (A – dorsal metasoma, B- ventral metasoma), 317.38 [M+7Li]+ (C22) (C – dorsal metasoma, D - ventral metasoma), 345.41 [M+7Li]+ (C24) (E – dorsal metasoma, F- ventral metasoma) and 359.42 [M+7Li]+ (C25) (G – dorsal metasoma, H - ventral metasoma). Optical images of dissected dorsal-terga (I) and ventral-sterna metasomas (J) of queen.
Discussion
This study provides the first evidence of a queen signal that regulates the activation of ovaries in stingless bee workers. An extract of the cuticular compounds from the surface of a single F. schrottkyi queen is sufficient to inhibit worker ovary activation in queenless workers for over 10 days.
Queens use cuticular hydrocarbons to signal their presence and inhibit worker ovary activation in diverse Hymenopteran social insects including ants2,5, wasps2, bumble bees2 and stingless bees (this study). Although one class of compounds (long chain alkanes) is strikingly similar across widely diverged taxa, the active compound can vary between groups. For example workers of the ant Lasius niger respond to 3-methylhentriacontane (methyl branched C31) and it alone is sufficient to reduce the activation of worker ovaries5.
The primary function of cuticular hydrocarbons is protection against desiccation and they are therefore present in all insects20. Changes in the cuticular hydrocarbon profile likely occur as an epiphenomenon of oogenesis and therefore provide an ‘honest signal’26 of a female's fertility. Thus cuticular hydrocarbons were repeatedly co-opted as the queen signal as eusociality independently emerged in the Hymenoptera2.
Remarkably, the structure and source of queen pheromones of the 12 species of honey bees differ greatly from other social insects, including their sister tribes the stingless bees and bumble bees7 and are not based on cuticular hydrocarbons. Most of the honey bee queen's signal is produced in the mandibular gland and is a complex blend of ketones and fatty acids27. Why the honey bee queen's signal should be so different to all other social insects is perplexing and warrants further investigation.
Worker antennae responded to the first ten peaks in the list of queen compounds, which are the most volatile compounds. The lack of response to longer chain hydrocarbons might be a limitation of the EAD test, since its response detection is conditional on the volatility of the compound under test. Thus, there may be compounds additional to those described here that play a role in queen-worker interactions. Nonetheless, EAD technology has permitted us to confirm worker responses toward queen cuticular compounds and allowed us to identify some key candidate compounds for the spatial distribution part of our study. We have shown that the cuticular hydrocarbons have differential distribution on the head and body of the queen (Table S2). The C21 alkane is the most abundant hydrocarbon detected in the head and it is distributed over the front part of the head. This distribution suggests that workers respond to this compound during the ritualised process of brood cell provisioning and oviposition process since the queen's head is constantly exposed to workers, which repeatedly contact the head region with their antennae until food provisioning commences13,14.
On the metasoma, the ions m/z 303, 317 and 345 (C21, 11-MeC21 and C24, respectively) showed higher ion intensities on the dorsal than ventral surface, with increasing intensity towards the distal end of the abdomen. This distribution may indicate that the source of the queen pheromone is from the integument glands of the abdomen or the reproductive glands such as the Dufour's gland17.
The behaviour of stingless bee queens suggests the tip of the abdomen as a source of chemical signalling. Workers are intensely attracted towards the abdomen of recently- emerged virgin queens. Young virgins patrol the nest and use their abdomen to hit aggressive workers28,29. A worker that has been hit by the queen's abdomen typically engages in self-grooming and the aggressiveness that is typically directed towards new queens gradually decreases28,29. The specific distribution of active compounds in this body region corroborates the idea of a chemical mediation of the interactions between virgin queens and workers. Furthermore, the hydrocarbon profile of the Dufour's gland matches the queen's cuticle profile in the stingless bee Melipona bicolor30,31, providing further support for the hypothesis that the Dufour's gland is a source of some of the queen's signal in stingless bees.
A C25 alkane is the most abundant compound on the F. schrottkyi queen's body11. Its relative concentration on the head is low relative to the rest of the body (Table S2, Supplementary Information). The distribution of this compound on the queen's metasoma shows a different pattern to the other active compounds because C25 appears to be concentrated on T4 (Fig. 3G). This could indicate that T4 is the site of secretion of C25, or a contamination with compounds present on the wings since the wings make contact with the abdomen at this point.
The cuticular hydrocarbon profile of workers and queens has been described for the stingless bees Frieseomelitta varia32, Schwarziana quadripunctata33 and Melipona bicolor31. Queen/worker differences are found in species where the workers are completely sterile (e.g. F. varia32), species where workers activate their ovaries and lay trophic eggs but do not contribute to male production (S. quadripunctata33,34) and species where reproductive workers contribute to male production even in the queen's presence (M. bicolor31,34). Examination of chemical cuticular profiles of stingless bee workers within a phylogenetic framework showed that both genetic and environmental factors shape the chemical profiles of stingless bees35. Similarly, a systematic investigation of worker reproduction in stingless bees and the variation of the cuticular compounds of queens and workers in a phylogenetic context will help to elucidate the evolution of queen-worker signals and reproductive conflicts within this group.
Methods
Suppression of worker ovary activation through queen cuticular extracts
F. schrottkyi colonies were obtained from the wild and transferred to plastic hives 4.0 × 5.0 × 10.0 cm. Each hive was covered by a glass lid, maintained in a laboratory and connected to the exterior of the building via an individual plastic tube. The colonies were randomly assigned to one of three treatments: (1) Control (queenright), (2) Queenless and (3) Queen extract (n = 7 colonies per treatment). The control colonies allowed us to assess the background level of worker ovary activation. In the two remaining groups the queen was removed from the colony at the beginning of the experiment and replaced later in the day by a glass bead covered with a hexane cuticular extract of the original queen or hexane wash (queenless treatment)11. The whole queen extracts were transferred to the surface of the glass beads (3 mm diameter) by suspending the beads on cotton line and depositing the hexane extracts onto each bead one drop at a time with complete evaporation of the solvent between drops. The glass beads were placed on top of newly-constructed brood comb.
Middle-aged workers are able to activate their ovaries and lay eggs less than ten days after removal of their colony's queen36. Ten days after we removed the queens from our colonies we sampled about 12 middle-aged (based on their cuticular colour and activity on the brood comb) workers (11.7 ± 1.7, mean ± SD, n = 246) from each colony (see supplementary information for the colours selected). We dissected these workers under 20 times magnification to evaluate their degree of ovary activation. One person who was unaware of the experimental treatment of the samples performed all the dissections. Ovaries were classified as inactive when there were small ovarioles with no oocytes and activated when an oocyte was present at any stage of development in at least one of the two ovarioles.
Data were analysed using the glmer function from the lme4 package37. We used a generalized linear mixed model (GLMM) with binomial error distribution to assess differences (α < 0.05) among treatments. Ovary activation (activated or inactivated) was used as the dependent variable and treatment (with three levels: control, queenless, queen extract) as categorical independent variable. Individual workers were regarded as repeated measures within colonies, which were regarded as a random factor.
Electroantennography test
We obtained three F. schrottkyi queens and killed them by freezing. The body of each queen was extracted in 300 µL hexane for 5 minutes. Two F. schrottkyi worker antennae were excised and attached to an electrode of a Syntech electroantennographic detector (Hilversum, Netherlands) with electrically-conductive gel. Antennae were then exposed to the eluent as queen extract was passed through a gas chromatograph (Shimadzu GC2010 with a flame-ionization detector). The electrical responses of the antennae were recorded and correlated with the chromotograph peaks (GC-EAD Syntech software, v. 4.6). Response to particular hydrocarbons was confirmed using synthetic analogues. Additional information concerning the electroantennographic tests are described in the supplementary material.
Synthesis of lithium 2,4-dihydroxybenzoate (LiDHB)
2,4-dihydroxybenzoic acid (0.507 g) was solubilized in ethanol (2 mL), added LiOH (0.077 g) and 2 mL of ultrapure water. The mixture was then heated (60°C, 1 hour) while being agitated by continuous stirring. Subsequently, the solution was concentrated in a rotatory evaporator. The crystals were washed with ice-cold ethanol and dried.
MALDI-MS analyses of standards
The LiDHB matrix was prepared at concentration 20 mg/mL in chloroform and acetone (3:7), which was added onto a ground stainless steel MALDI target (1 µL). Subsequently the standard solutions (1 µL) were added on the matrix. The commercial and synthetized standards were solubilized in chloroform and acetone (3:7). The external calibration was conducted with a mixture of flavonoids (galangin, quercetin, rutin and isoquercitrin). The MS parameters applied were the following: 1000 Hz - laser frequency, 100 ns - pulsed ion extraction, reflectron positive mode and 2000 shots (500 shots per position) were acquired to get a spectrum.
Spatial distribution of cuticular compounds by MALDI imaging
Queen cuticle was carefully removed avoiding destruction of the wax layer. The tissues (head – frontal and posterior parts and ventral and dorsal metasomas) were affixed to indium tin oxide-coated conductive slides (ITO, Bruker Daltonics) using double-sided tape (3M Co., USA). LiDHB matrix solution was prepared at 20 mg/mL in chloroform and acetone (3:7). The matrix was applied in the tissues using an ImagePrep work station (Bruker Daltonics) and dried under a stream of nitrogen. The analyses were performed in an MALDI-TOF/TOF UltrafleXtreme (Bruker Daltonics, Bremen, Germany) instrument. The following parameters were used to acquire the images: 110 ns PIE, 1000 Hz laser frequency, reflector positive mode, 500 shots. All spectra were internally calibrated with the ions from the matrix (m/z 251 and 351). The images were produced using the software FlexImaging 2.1 (Bruker) at 40 µm spatial resolution in both x and y directions.
Synthesis of queen cuticular compounds
Solvents were purified according to standard procedures38. All air-sensitive and/or water-sensitive reactions were carried out with dry solvents under anhydrous conditions and a nitrogen atmosphere. Standard syringe techniques were applied for the transfer of dry solvents and air-sensitive reagents. The reactions were monitored by TLC on Merck silica gel (60 F 254) visualized with 5% vanillin in 10% H2SO4 with heating as a developing agent. The synthesized compounds were submitted to flash column chromatography (Sigma–Aldrich silica gel -particle size 0.040–0.063 nm) using hexane as eluent. The purified compounds were analysed by GC/MS (Shimadzu GCMS-QP2010) and NMR (see Supporting Information). For the synthesis of compounds 6a-c, initially the intermediate 1-bromoundecane (2) was synthetized, then the triphenyl(undecyl)phosphonium bromide (3) and the alkenes 5a-c were also synthetized, which were used to produce the branched hydrocarbons 6a-c39. The general pathway to the synthesis of the target compounds are illustrated in Fig. 4. All the steps of synthesis and data, including NMR data, are described in Supplementary Information.
Synthesis of 11-MeC21, 11-MeC23 and 11-MeC25.
Commercially available undecyl alcohol 1 (Aldrich, Stanheim, Germany) was converted to the bromide 2 and, subsequently, to the Wittig salt 3 by using triphenylphosphine in dry toluene. The salt 3 was then methylated at the α-position using n-butyl lithium and methyl iodide and submitted to a sequential Wittig reaction with commercially available aldehydes 4a and 4b and aldehyde 4c (previously synthesized from tetradecan-1-ol by PCC oxidation). The resulting alkenes (5a, 5b and 5c) were hydrogenated to yield the target 11-methylated alkanes 6a, 6b and 6c.
References
Hoover, S. E. R., Keeling, C. I., Winston, M. L. & Slessor, K. N. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften 90, 477–480 (2003).
Van Oystaeyen, A. et al. Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287–290 (2014).
Keeling, C. I., Slessor, K. N., Higo, H. A. & Winston, M. L. New components of the honey bee (Apis mellifera L.) queen retinue pheromone. PNAS 100, 4486–4491 (2003).
Winston, M. L., Higo, H. A. & Slessor, K. N. Effect of various dosages of queen mandibular gland pheromone on the inhibition of queen rearing in the honey bee (Hymenoptera: Apidae). Ann. Entomol. Soc. Am. 83, 234–238 (1990).
Holman, L., Jørgensen, C. G., Nielsen, J. & d'Ettorre, P. Identification of an ant queen pheromone regulating worker sterility. P. Roy. Soc. B-Biol. Sci. 277, 3793–3800 (2010).
Michener, C. D. The bees of the world. (John Hopkins University Press, 2000).
Thompson, G. J. & Oldroyd, B. P. Evaluating hypotheses on the origin of eusociality in corbiculate bees. Mol. Phylogenet. Evol. 33, 452–456 (2004).
Michener, C. D. The social behavior of the bees. (Harvard University Press, 1974).
Palmer, K. A., Oldroyd, B. P., Quezada-Euán, J. J. V., Paxton, R. J. & May-Itza, W. D. Paternity frequency and maternity of males in some stingless bee species. Mol. Ecol. 11, 2107–2113 (2002).
Sakagami, S. F., Camillo, C. & Zucchi, R. Oviposition behavior of a Brazilian stingless bee Plebeia (Friesella) schrottkyi, with some remarks on the behavioral evolution in stingless bees. J. Fac. Sci. Hokkaido U. Zool. 19, 361–421 (1973).
Nunes, T. M., Morgan, E. D., Drijfhout, F. P. & Zucchi, R. Caste-specific cuticular lipids in the stingless bee Friesella schrottkyi. Apidologie 41, 579–588 (2010).
Drumond, P. M., Oldroyd, B. P., Dollin, A. E. & Dollin, L. J. Oviposition behaviour of two Australian stingless bees, Austroplebeia symei Rayment and Austroplebeia australis Friese (Hymenoptera: Apidae: Meliponini). Aust. J. Entomol. 38, 234–241 (1999).
Zucchi, R. [Ritualized dominance, evolution of queen-worker interactions and related aspects in stingless bees (Hymenoptera: Apidae)]. Evolution of insect societies [Inoue, T. & Yamane, S. (eds.)] [207–249] (Hakuhinsha, Tokyo, 1993).
Zucchi, R., da Silva-Matos, E. V., Nogueira-Ferreira, F. H. & Azevedo, G. C. On the cell provisioning and oviposition process (POP) of the stingless bees - Nomenclature reappraisal and evolutionary considerations (Hymenoptera, Apidae, Meliponinae). Sociobiology 34, 65–86 (1999).
Sakagami, S. F. in Social Insects Vol. III (ed Hermann, H. R.) 361–423 (Academic Press, 1982).
Cruz-Landim, C. & Abdalla, F. C. Glândulas exócrinas das abelhas. [Cruz-Landim, C. & Abdalla, F. C. (eds.)](FUNPEC, Ribeirão Preto, 2002).
Cruz-Landim, C. Abelhas: morfologia e função de sistemas. (Editora UNESP, São Paulo, 2009).
Oldham, N. J., Billen, J. & Morgan, E. D. On the similarity of the Dufour gland secretion and the cuticular hydrocarbons of some bumblebees. Physiol. Entomol. 19, 115–123 (1994).
Kather, R. & Martin, S. J. Cuticular hydrocarbon profiles as a taxonomic tool: advantages, limitations and technical aspects. Physiol. Entomol. 37, 25–32 (2012).
Blomquist, G. J. & Bagnères, A.-G. Insect hydrocarbons: Biology, Biochemistry and Chemical Ecology [Blomquist, G. J. & Bagnères, A.-G. (eds.)] (Cambridge University Press, 2010).
Cvačka, J., Jiroš, P., Šobotník, J., Hanus, R. & Svatoš, A. Analysis of insect cuticular hydrocarbons using matrix-assisted laser desorption/ionization mass spectrometry. J. Chem. Ecol. 32, 409–434 (2006).
Cvačka, J. & Svatoš, A. Matrix-assisted laser desorption/ionization analysis of lipids and high molecular weight hydrocarbons with lithium 2, 5-dihydroxybenzoate matrix. Rapid Commun. Mass Spectrom. 17, 2203–2207 (2003).
Vrkoslav, V., Muck, A., Cvačka, J. & Svatoš, A. MALDI imaging of neutral cuticular lipids in insects and plants. J. Am. Soc. Mass Spectr. 21, 220–231 (2010).
Silva, D. B. et al. Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in lychnophora species. Sci. Rep. 4 (2014).
Gode, D. & Volmer, D. A. Lipid imaging by mass spectrometry–a review. Analyst 138, 1289–1315 (2013).
Keller, L. & Nonacs, P. The role of queen pheromones in social insects: queen control or queen signal? Anim. Behav. 45, 787–794 (1993).
Plettner, E. et al. Species- and caste-determined mandibular gland signals in honeybees (Apis). J. Chem. Ecol. 23, 363–377 (1997).
Imperatriz-Fonseca, V. L. & Zucchi, R. Virgin queens in stingless bee (Apidae, Meliponinae) colonies: a review. Apidologie 26, 231–244 (1995).
Jarau, S., Van Veen, J. W., Aguilar, I. & Ayasse, M. A scientific note on virgin queen acceptance in stingless bees: evidence for the importance of queen aggression. Apidologie 41, 38–39 (2010).
Abdalla, F. C., Jones, G. R., Morgan, D. & Cruz-Landim, C. d. Chemical composition of the Dufour gland secretion in queens of Melipona bicolor (Hymenoptera, Meliponini). J. Brazil. Chem. Soc. 15, 621–625 (2004).
Abdalla, F. C., Jones, G. R., Morgan, E. D. & da Cruz-Landim, C. Comparative study of the cuticular hydrocarbon composition of Melipona bicolor Lepeletier, 1836 (Hymenoptera, Meliponini) workers and queens. Genet. Mol. Res. 2, 191–199 (2003).
Nunes, T. M., Turatti, I. C., Lopes, N. P. & Zucchi, R. Chemical signals in the stingless bee, Frieseomelitta varia, indicate caste, gender, age and reproductive status. J. Chem. Ecol. 35, 1172–1180 (2009).
Nunes, T. et al. Cuticular hydrocarbons in the stingless bee Schwarziana quadripunctata (Hymenoptera, Apidae, Meliponini): differences between colonies, castes and age. Genet. Mol. Res. 8, 589–595 (2009).
Tóth, E., Strassmann, J. E., Imperatriz-Fonseca, V. L. & Queller, D. C. Queens, not workers, produce the males in the stingless bee Schwarziana quadripunctata quadripunctata. Anim. Behav. 66, 359–368 (2003).
Leonhardt, S. D., Rasmussen, C. & Schmitt, T. Genes versus environment: geography and phylogenetic relationships shape the chemical profiles of stingless bees on a global scale. P. Roy. Soc. B-Biol. Sci. 280, 20130680 (2013).
da Cruz-Landim, C. Ovarian development in Meliponine bees (Hymenoptera : Apidae): the effect of queen presence and food on worker ovary development and egg production. Genet. Mol. Biol. 23, 83–88 (2000).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. (2013). Available at: http://www.R-project.org/. (Accessed: 10th November 2014).
Armarego, W. L. & Chai, C. Purification of laboratory chemicals. (Butterworth-Heinemann, 2012).
Little, R. D. & Muller, G. W. Intramolecular diyl trapping. Total synthesis of dl-hirsutene. J. Am. Chem. Soc. 103, 2744–2749 (1981).
Acknowledgements
The authors are grateful to Alcemar, Alcemir and Alcindo de Andrade from Estância Boa Vista for providing biological material. This research was supported by FAPESP (TMN Proc. 2007/59058, 2011/22991-3; DBS Proc. 2012/18031-7; NPL Proc. 2009/54098-6 and 2014/20302-4 and RS 2014/01884-2) and CNPq/INCT_if.
Author information
Authors and Affiliations
Contributions
T.M.N., R.Z. and N.P.L. conceived and designed the research; S.M., T.M.N. and L.G.Z. collected the biological samples and designed the behavioural tests. L.G.Z. applied the statistical tests. S.M., dissected workers and queens. A.P.F., T.M.N. and J.M.S.B. designed and performed the electrophysiological tests. M.F.Z.J.A., T.M.N. and G.C.C. designed a synthetic pathway and synthesized the organic compounds. R.S., D.B.S. and N.P.L. conducted the MALDI imaging experiments. B.P.O., T.M.N., D.B.S. and N.P.L. wrote the manuscript and the other authors revised the paper.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Methodology details on the manuscript
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Nunes, T., Mateus, S., Favaris, A. et al. Queen signals in a stingless bee: suppression of worker ovary activation and spatial distribution of active compounds. Sci Rep 4, 7449 (2014). https://doi.org/10.1038/srep07449
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07449
- Springer Nature Limited
This article is cited by
-
The queens of the stingless bees: from egg to adult
Insectes Sociaux (2023)
-
Chemical Resemblance of Egg Surface Compounds and Dufour’s Gland in Two Neotropical Polistinae Wasps Polistes versicolor (Olivier) and Mischocyttarus metathoracicus (de Saussure, 1854)
Neotropical Entomology (2023)
-
Functional properties of ant queen pheromones as revealed by behavioral experiments
Behavioral Ecology and Sociobiology (2023)
-
Behavioral differentiation among workers may reduce reproductive conflicts during colony inheritance in the termite Reticulitermes labralis
Insectes Sociaux (2022)
-
Honeybee queen mandibular pheromone fails to regulate ovary activation in the common wasp
Journal of Comparative Physiology A (2022)