Abstract
Wearable enzyme-based biosensors enable advanced healthcare diagnostics through the monitoring of biomarkers and physiological states. The integration of materials engineering and enzyme conjugation has established the groundwork for advancements in modern analytical chemistry, poised to extend the frontiers of wearable biosensing further. Recent advancements in enzymatic biofuel cells have also enhanced devices by harnessing biofuels, such as glucose and lactate in biofluids. Importantly, biofuel cells offer the potential for self-powered biosensors. Here, we present an overview of the principles and considerations associated with engineering materials and integrating enzymes with electrodes to achieve effective wearable biosensing and self-sustaining biofuel cell-based energy systems. Furthermore, we discuss challenges encountered by enzymatic sensors and biofuel cells. Representative applications of wearable devices in healthcare settings are highlighted, along with a summary of real sample analyses, emphasizing the concentration ranges of analytes present in actual sweat samples to underscore their relevance in real-world scenarios. Finally, the discussion explores the anticipated impact of future material innovations and integrations on the development of next-generation wearable biodevices.
Similar content being viewed by others
Introduction
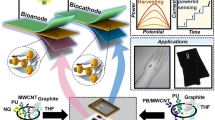
Wearable sensors, integrated analytical devices for analyzing biological fluids, have garnered significant interest in recent years due to their unique capability for real-time and continuous monitoring of biomarkers in a noninvasive or minimally invasive manner1,2. These sensing devices have been developed in diverse forms, such as temporary tattoos3,4, wristbands5,6, bandages7,8, textiles9,10, face masks11,12, eyeglasses13,14, contact lenses15,16, mouthguard17,18 and microneedles19, enabling access to biofluids for on-body analysis. In addition, recent advances in wearable sensors have demonstrated the effectiveness of real-time wireless monitoring20. The concept of wearable sensors enabling a digital diagnosis system is shown in Fig. 1a. Historically, traditional biosensors were constructed by immobilizing biorecognition elements on sensing interfaces. For instance, a glucose biosensor could be created by immobilizing glucose oxidase (GOx) on a carbon-based electrode to provide a selective recognition layer that specifically catalyzes glucose and converts biochemical concentration into a measurable signal on the electrode transducer21,22. This review focuses on enzymes for wearable healthcare sensing applications, prioritizing their widespread utility and significance, despite the presence of various other biorecognition approaches, e.g., antigen-antibody interactions23,24 and aptamer recognitions25,26. Various engineered materials have also played a role in enhancing sensing performances by controlling properties such as conductivity, surface area, and catalytic activity of electrodes27,28,29. However, materials for traditional off-body sensors were primarily designed to enhance analytical performance without considering crucial features such as biocompatibility, flexibility, wearability, and comfort. Furthermore, the operation of traditional analysis often entailed system complexity, reliance on external energy sources, pH value adjustments, and pre-treatment of biofluid samples. These representative aspects must be considered when developing materials and enzymatic electrodes for practical applications.
a Wearable sensors enabling digital diagnosis system. b Illustration demonstrating integration of functional materials with enzymes for biorecognition to detect biomolecule targets, e.g., glucose and lactic acid. c A typical amperogram from a biosensor, accompanied by the calibration curve utilizing amperometric current as a signal. d Illustration showing the working principle of a wearable biofuel cell-based self-powered biosensor. e Power-voltage curve obtained from the biofuel cell-based device, accompanied by the calibration curve utilizing self-generated power or current as a signal.
Alongside the advancement of biosensing layers via the coupling of biorecognition elements with transducing materials, another essential consideration pertains to the provision of energy for the entire functional wearable system. This is particularly crucial for wearable devices aiming for a compact and standalone platform for uninterrupted monitoring. Therefore, strategies for achieving self-sustaining energy systems to integrate with wearable sensors are being explored30,31,32. Among many energy-harvesting alternatives from the human body, enzymatic biofuel cells (EBFCs) hold promise for harnessing energy from sweat fuel sources containing metabolites (such as glucose and lactate)33,34. EBFCs also offer the unique capability to act as self-powered biosensor units, simplifying the system by merging the sensing unit with the energy unit35. To achieve desirable wearable biosensing and self-sustaining systems through enzyme-material integration, optimizing engineering materials is crucial. This optimization should tackle challenges such as the rigidity and bulkiness of traditional devices, poor stability, slow electron transfer, biofouling and interfering effects from complex samples, and limitations in surface area and enzyme immobilization, substrate availability, and oxygen concentration.
Several recent review articles have explored various topics, including wearable devices36, point-of-care biosensors37, biofuel cells38, and advanced materials for electrochemical biosensors39,40. For example, one published review discusses the fabrication of wearable biofuel cells, exploring the materials and strategies applicable to their construction41. Additionally, it explores the diverse applications of these biofuel cells, summarizes and compares the materials and energy performances of various wearable biofuel cells, and suggests strategies for enhancing their performance. Similarly, other reviews have addressed the fabrication and applications of wearable biofuel cells42,43. The published reviews also cover self-powered wearable sensors, energy harvesters, energy storage, and power supply strategies44. Our review, however, focuses on biosensing applications for health monitoring using wearable enzyme-based and self-powered devices. We also aim to contribute additional thought-provoking perspectives and further assimilate information pertaining to updated trends in these emerging areas. Our review delves into approaches to engineering materials and enzyme-material integration to achieve high-performance wearable biosensing and self-sustaining biosensing devices. Key strategies to address challenges posed by engineering materials for enzyme conjugation in biorecognition for wearable biosensing and self-sustaining energy systems are explored. Aspects regarding biocompatibility to preserve enzymatic activity, ensuring rapid communication between immobilized enzymes and electrodes, and providing mechanical flexibility to conform to the body in wearable devices are addressed. Moreover, durability considerations address the need for sustained performance in real-world scenarios. Representative settings are highlighted, along with an overview of real sample analyses, emphasizing the concentration ranges of analytes, including sweat glucose and lactate. It emphasizes the importance of on-body settings for obtaining comprehensive results, particularly utilizing multiplex sensors to analyze actual analyte concentrations in real sweat samples. This review explores wearable systems capable of simultaneously analyzing various significant biomarkers, including glucose, lactate, uric acid, Na+, K+, NH4+, pH, and physiological signals such as temperature, electrocardiogram (ECG), electroencephalogram (EEG), and electromyography (EMG), to provide insights into health conditions. In addition to discussing diagnostic systems, this review also presents representative examples of integrated drug-releasing systems. This integration illustrates the advancement of closed-loop biomedical systems that incorporate diagnosis and therapy. Additionally, the outlook section discusses the potential impact of future material integrations and strategies on the development of next-generation wearable biodevices.
Underpinning the use of advanced functional materials for enzyme conjugation in biorecognition for biosensing applications
Advanced functional materials are crucial in enzyme-based biosensors. Engineering features such as surface functionality, shape, surface area, and composition enable the tailoring of characteristics to enhance performance in supporting sensor development45. Enzymes have been immobilized within nanostructured materials, such as nanoparticles and nanotubes, through covalent binding, adsorption, and entrapment techniques46,47, thereby facilitating the development of sensitive biosensors. Materials, such as carbon nanomaterials, metal nanomaterials, and conductive polymers, have been conjugated with enzymes to ensure the high performance of point-of-care sensors due to their high electrical conductivity and high surface area-to-volume ratio. They offer advantages in facilitating rapid electron transfer between enzyme active sites and electrode surfaces, as well as enhancing catalytic properties and sensing responses48.
Optimizing wearable enzyme-based electrodes requires careful consideration of various factors related to the structural and chemical design of materials. Key considerations encompass the selection of materials and structures to manage surface characteristics, biocompatibility, porosity, and surface functionalization. For example, nanomaterials with high surface area enhance enzyme immobilization by maximizing active sites and promoting close enzyme-electrode proximity for efficient electron transfer49,50. Moreover, fabricating nanostructures, such as porous architectures, can facilitate efficient mass transport of analytes to enzyme active sites, promoting rapid diffusion of target molecules to the sensor surface and improving response time51,52. Additionally, nanocomposite materials comprising carbon materials and precious metal nanoparticles can enhance both electron transfer and catalytic activity53,54. Furthermore, stability and activity can be tuned through engineering nanomaterials and enzyme-material integration strategies to improve immobilization efficiency, thereby expanding the long-term and analytical performances of on-body sensors.
Incorporating energy management aspects into wearable devices is also crucial for their functionality. The combination of energy harvesting and storage elements within wearable biosensor platforms facilitates self-sustained monitoring44. A self-sustaining system primarily comprises various energy devices, including energy-harvesting and energy-storage components such as self-charging biosupercapacitors12, alongside biosensors capable of harnessing power from biofluids by energy-harvesting devices (such as EBFCs). These devices are designed to ensure the full system is self-sustaining, thereby provisioning a continuous power source for the sustained operation of wearable biosensors. The synergistic interplay between sensing and energy device functionalities yields autonomous devices ideal for practical platforms.
Principles of wearable enzyme-based biosensors and self-powered biosensors
Enzymes play a role in biosensors due to their specificity and catalytic activity, enabling the selective and sensitive detection of analytes55,56. Immobilizing enzymes onto wearable electrodes allows biosensors to convert biochemical reactions into measurable electrical signals, facilitating real-time monitoring of chemicals57. Various electrochemical techniques, such as amperometry, capitalize on this enzyme-immobilized electrode setup to translate biochemical concentrations into corresponding electrical parameters58. For example, one of the most widely used principles relies on amperometry coupled with immobilized oxidase enzymes on functional electrodes. These enzymes catalyze the oxidation of analytes, such as glucose59,60. The resulting oxidation event or the indirect measurement of the catalytic reaction product, such as H2O2, can be detected by the electrochemical transducer using the amperometric technique (Fig. 1b). Consequently, the concentration of analyte can be detected by the current increment observed in current-time curves (Fig. 1c). Other techniques beyond amperometry are also viable. Impedance techniques assess changes in electrode surface properties caused by enzyme-substrate interactions61, while voltammetry applies varying potentials to the wearable electrode to induce redox reactions, offering analytical insights into analyte concentrations62. Potentiometry measures the potential difference generated during enzymatic reactions63. These examples provide versatile tools for wearable biosensing applications. Nanomaterial-based biosensors on wearable electrodes have garnered attention for enhancing electrochemical signals and effectively tethering enzymes64. Nanomaterials generally improve the surface area, conductivity, and electron-transfer ability of the sensing interface.
Energy efficiency is particularly crucial for wearable monitoring, necessitating standalone and compact biosensors capable of continuous signal detection65,66. Integrating energy harvesting and storage devices with wearable biosensors is essential, making EBFCs appealing for self-powered bioelectronics33,35. As shown in Fig. 1d, these cells utilize enzymes as electrocatalysts to catalyze reactions converting fuel (analyte) into electricity, similar to conventional fuel cells. In an EBFC, an enzyme facilitates the oxidation of fuel at the bioanode, liberating electrons that flow to the cathode via an external circuit. Meanwhile, at the cathode, oxygen (O2) is reduced, completing the circuit cycle and thus generating an electrical current67. EBFCs boast advantages such as renewable biocatalysts and compatibility with various fuels, including sugar and alcohol.
This working principle can be leveraged to create wearable EBFCs to harness biochemical energy from bodily fluids, thereby facilitating the development of power-harvesting devices for self-sustainable in vivo applications and miniaturized sensors. EBFCs are instrumental in powering self-sustaining devices, lessening dependence on conventional batteries, and ensuring uninterrupted analysis68. By enzymatically oxidizing biofuels at the bioanode and accepting electrons at the cathode, EBFCs produce power corresponding to the biofuel (or analyte) concentration (Fig. 1e)69. This self-sustaining energy model enhances the sustainability and convenience of biosensing devices.
Engineering materials and designs for wearable enzyme-based biosensors and self-powered enzyme-based biosensors
Transitioning to biosensors for detecting biochemicals requires integrating materials and enzymes, posing significant challenges due to the numerous interfaces involved. These interfaces include those between electrodes and enzymes, as well as between immobilized enzymes and biofluid samples in dynamic environments. Challenges encompass material flexibility, electrode nanostructure, stability of enzyme and mediator immobilization, electron transport efficiency, substrate and O2 availability, and biocompatibility. One major challenge often results in low or fluctuating current outputs, such as low amperometric currents in enzymatic biosensors or low power outputs in EBFCs. Despite efforts, wearable enzymatic sensors and EBFCs still face performance issues, including lower current or power densities, reduced volumetric catalytic activity, low energy density, or open circuit voltage. Several designs have been proposed for long-term monitoring devices with a focus on operational duration. Monitoring results must be reliable and promptly delivered, while sensors should be universally accessible and user-friendly with a high frequency of readings. There is a demand for user-friendly long-term monitoring devices that prioritize miniaturization, biocompatibility, and minimal interference with daily activities. Wearable sensors are particularly valued for such applications70. An example is a small sweat sensor with a dimension of approximately 1.5 cm × 1.5 cm, which demonstrates size reduction and enhanced wearing comfort71. Hence, it is crucial to consider multiple strategies to mitigate these challenges and meet the requirements for achieving high performance in wearable enzymatic biosensors and EBFC-based self-powered sensors.
Exploring mechanical properties considerations
Wearable devices require flexibility to withstand dynamic deformations caused by human body movements. Engineering materials to mitigate rigidity, which does not align with the softness of human tissues, present considerable challenges while preserving essential functions such as high conductivity and efficient electron transfer. Employing soft materials facilitates the creation of comfortable devices that adapt to the body’s movements. Moreover, opting for inexpensive and lightweight materials for miniaturized flexible sensors is necessary to address the need for practical fabrication and wearer comfort.
Polymers, e.g., polyurethane (PU), Ecoflex®, and polydimethylsiloxane (PDMS), are extensively utilized as substrates for sensors or as the polymer matrix in nanocomposites. Apart from their high elasticity, these materials exhibit biological compatibility72. The integration of polymers with conducting materials such as carbon materials73,74, metal nanoparticles75,76, or conductive polymers77,78 produces flexible electrodes. Stretchability can be realized by employing nanomaterial-based stretchable inks, blending carbon (e.g., CNTs) with the elastomer. For instance, a wearable textile-based sensor was fabricated by printing CNT ink electrodes on layers of Ecoflex® and PU to enhance the flexibility of the printed electrodes (Fig. 2a)79. This self-powered biosensor exhibited resilience against repeated mechanical deformations. Another example is a diabetes patch (Fig. 2b)80. This patch features a sensing array printed onto a silicone film, allowing for conformal contact with human skin.
a Textile-based EBFCs integrated with socks for self-powered lactate sensors, subjected to mechanical resilience tests during twisting deformations. Adapted with permission from ref. 79 © 2016, Royal Society of Chemistry. b Wearable diabetes monitoring utilizing silicon film attached to human skin. Adapted with permission from ref. 80 © 2016, Springer Nature Limited. c Printed devices for electrochemical sensors and EBFCs based on stretchable CNT-based ink. Adapted with permission from ref. 81. © 2016, American Chemical Society. d Skin-mountable wearable EBFC device with an “island-bridge” design capable of harvesting sweat energy to power a light-emitting diode (LED). Adapted with permission from ref. 82. © 2019, WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Mechanical engineering designs also enable the effective distribution of strain within the electrode structure. A printed biosensing device, featuring a stretchable CNT-based ink, exemplifies this concept (Fig. 2c)81. The use of a free-standing serpentine electrode enables substantial strain levels (up to 500%) to be achieved. The freestanding nature of bridges allows for more independent movement, thereby boosting stretchability.
Island-bridge electrode devices with freestanding serpentine bridges offer effective strain distribution. The inclusion of a serpentine pattern in the bridges allows for stretching deformation without excessively concentrating stress. Additionally, integrating a stretchable ink formula with an “island-bridge” design enhances the resilience of device. An example includes a skin-mountable wearable biofuel cell for lactate level monitoring, utilizing a layer of Ecoflex® (Fig. 2d)82. This design features carbon-based electrode “islands” connected by conductive silver “bridges” in a serpentine shape. Mechanical stability is ensured by incorporating a polystyrene (PS) “skeleton” and styrene-ethylene-butylene-styrene (SEBS) beneath the carbon islands.
Optimizing nanostructures for efficient enzyme-material integration
The electrical output of enzymatic sensors and EBFCs is often constrained by slow electron transfer, enzyme catalytic inefficiency, and limited mass transport. A key strategy to address such limitations involves engineering materials to achieve high surface area, such as porous electrodes. These materials enhance current density, facilitate mass transport, and increase enzyme loadings. For instance, a nanoporous carbon-encapsulated 3D porous graphene electrode boosts electrochemical surface area and electrocatalytic activity (Fig. 3a)83. The structure of the pore is important when dealing with enzymes. For example, pore mouths that are too small (< 3.5 nm) make GOx adsorption difficult84. Laser-induced graphene, produced by directing a CO2 IR laser at a polyimide film is another way to achieve a large surface area85. Nanoporous gold electrode (NPG) also provides similar advantages; for example, NPG (with pores around 21 nm) enables high enzyme loadings and enhanced current density (Fig. 3b)86. The fabrication starts with magnetron sputtering gold/silver alloy. Subsequently, electrochemical dealloying of silver was conducted in solutions (NaF and sulfuric acid) to achieve NPG.
a Porous carbon-reinforced 3D graphene electrode showcasing enhanced surface area and electrocatalytic activity. Adapted with permission from ref. 83. © 2022, Elsevier B.V. b Porous gold-based EBFCs integrated onto contact lenses. Adapted with permission from ref. 86. © 2018, American Chemical Society. c Wearable epidermal biofuel cells utilizing 3D coralloid nitrogen-doped hierarchical-micromesoporous carbon aerogels. Adapted with permission from ref. 87. © 2021, Elsevier Ltd.
In EBFCs, both the anode and cathode should be optimized to maximize fuel transport to the enzyme’s redox-active site. By employing porous nanostructured materials in EBFCs, power densities can be elevated from μW cm−2 to mW cm−2. An example is the 3D coralloid nitrogen-doped carbon aerogels (with pore sizes ranging from 0.5 to 4 nm) exhibiting a high specific surface area of ~ 500 m2 g−1 (Fig. 3c)87. These aerogels improve bioelectrode performance.
The high electrocatalytic NPG electrode can be used for ethanol and glucose biosensors to reduce the detection potential88. NPG with a pore size of ∼ 40 nm was also demonstrated to be a good carrier for biomacromolecules. The high density of edge-plane-like defective sites and large specific surface area of NPG also exhibited enhanced electrocatalysis toward reduced nicotinamide adenine dinucleotide (NADH) and H2O2.
Improving enzyme immobilization for controlling stability and electron transfer kinetics
The immobilization of enzymes on wearable electrode surfaces while preserving their activity presents a significant challenge. Example challenges include the low power density and limited lifetime in EBFCs. These limitations can hinder their application as self-powered biosensors due to reduced sensitivity. Moreover, enzyme deactivation and mass transfer barriers further impede enzyme activity, necessitating careful consideration during the design of immobilization strategies.
Common immobilization strategies include physical adsorption89 covalent binding90, entrapment91, or cross-linking92. Crosslinking in hydrogels is an example to enhance enzyme loading and analyte/biofuel transport efficiency93. Ensuring rapid electron transfer between enzymes and the electrode surface is also needed for optimizing the performance. This involves designing scaffolds for enzyme immobilization and developing effective enzymes or enzyme cascades with improved catalytic activity to fully harness the potential of enzymatic reactions94,95. Employing enzyme cascades can enhance the sequential oxidation of fuel, improving communication between consecutive enzymes, thereby increasing sensitivity or energy conversion96.
Using electron mediators enables rapid rates of electron transfer. Additionally, immobilizing enzymes on conductive materials with high specific surface areas can address challenges about low power density and short lifetimes. For example, a structure combining lactate oxidase and 1,4-naphthoquinone mediator into an MXene/single-walled carbon nanotube composite has been developed to harvest energy from sweat (Fig. 4a)97.
a MXene-based bioanode for wearable biosupercapacitor. Adapted with permission from ref. 97. © 2023, Wiley‐VCH GmbH. b EBFCs using layer-by-layer assembled GOx-coated metallic cotton fibers. Adapted under the terms and conditions of CC-BY 4.098, © 2018, Cheong Hoon Kwon et al., published by Springer Nature. c A glucose EBFC based 3D bioelectrodes. Adapted with permission from ref. 99. © 2013, Royal Society of Chemistry.
Another example is the fabrication of a porous metallic cotton fiber (MCF)-based EBFC using a layer-by-layer assembly method. This approach enhances the direct electron transfer (DET) rate between enzymes and conductive supports (Fig. 4b)98. In this setup, MCFs serve not only as a conductive host for depositing anodic enzyme materials but also as electrocatalytic cathodes for oxygen reduction reactions (ORR). Multilayers composed of GOx and tris-(2-aminoethyl)amine facilitate electron transfer through the enzyme layers.
Combining DET and mediated electron transfer (MET) offers another avenue to boost electron transfer rates in EBFCs. For instance, a glucose EBFC utilizing naphthoquinone-mediated glucose oxidation by GOx and direct oxygen reduction by laccase was demonstrated. This configuration achieves a high open-circuit voltage of 0.76 V, current densities of approximately 4.5 mA cm−2, and a maximum power output of about 1.6 mW cm−2 (Fig. 4c)99.
Considering enzyme orientation is crucial when integrating enzymes with materials. Regulating enzyme orientation and electron transfer on functional electrodes can be achieved by designing a redox mediator including an aromatic site for enzyme orientation, an anchoring site intended for immobilization, and a redox mediator for electron transfer100. This is realized through the development of a redox mediator containing a pyrene group to regulate enzyme orientation, an electron-carrier redox mediator (2,2’-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS)), and an electropolymerizable pyrrole. The bioelectrode modified with pyrrole–ABTS–pyrene groups demonstrates favorable performance in constructing an EBFC.
Maintaining enzyme stability is crucial. Enzymes are vulnerable to environmental factors such as temperature and pH, posing challenges in finding materials that can preserve enzyme activity over time. Reactive oxygen species (ROS), like O2‧−, generated during incomplete O2 reduction, can impact enzyme activity101. Additionally, instability may stem from enzyme or cofactor use. In addition to enzyme stability, the materials utilized for enzyme conjugation are crucial. Factors such as low stability of mediators, electrode contamination, or toxicity can also impose limitations on wearable operation. Combining biotechnology (through extremophilic enzymes102 and enzyme engineering103,104 with materials engineering strategies can enhance the chemical microenvironment for enzymes at electrode surfaces, thereby improving stability. For example, a porous membrane composed of emulsion-based agarose, glycerol, and aluminum oxide nanoparticles demonstrated enhanced sensing stability and reliable long-term monitoring for up to 20 h105.
Mitigating effects of interferences and biofouling
Analyzing complex biofluids poses a significant challenge. For instance, in glucose analysis, interference effects such as the co-oxidation of unwanted compounds (e.g., ascorbic acid, acetaminophen, and uric acid) can distort glucose concentration results. Biofluids also contain substantial molecules that impede electrochemical signals. This challenge is notable in on-body applications, where biofluids contain the target analyte and potential interfering and biofouling substances.
Enzymes facilitate reactions with precision, catalyzing specific transformations. Selecting the high specificity of enzymes, such as GOx, which catalyzes the oxidation of β-d-glucose to d-glucono-δ-lactone and H2O2 in the presence of O2, is essential for improving accuracy. Additionally, developing materials such as interference rejection membranes can further refine analytical performance within such complex environments. Various approaches can be employed to eliminate interferences106. One approach uses permselective membranes, such as negatively-charged perfluorinated ionomers (e.g., Nafion)107 and cellulose acetate18, to repel anionic interferents (e.g., ascorbic acid). Another approach is using mediators, such as ferrocene, methylene blue, and tetrathiafulvalene-tetracyanoquinodimethane, to lower the detection potential108. Catalysts (e.g., Prussian blue or horseradish peroxidase) can also facilitate the reduction of H2O2, thereby minimizing the detection potential to avoid overvoltage and false signals from interfering species109. Moreover, cross-linked poly(2-hydroxyethyl methacrylate) and cellulose acetate permselective membranes have been shown to eliminate interferences, such as ascorbic acid, uric acid, and acetaminophen18,110. Furthermore, electrostatic repulsion between graphene oxide and interferences, coupled with the electrocatalytic properties of the silver nanoparticles@multi-walled carbon nanotubes nanocomposite (AgNPs@MWCNTs), can eliminate ascorbic acid and uric acid interferences111.
To mitigate the biofouling effect, zwitterionic polymers based on methacryloyloxyethyl phosphoryl-choline polymer can be used for coatings to prevent protein adsorption and cell adhesion112. This is attributed to the formation of a strong hydration sphere113. Additionally, enzyme electrodes coated with poly(2-methacryloyloxyethyl phosphorylcholine-co-glycidyl methacrylate) exhibit higher glucose sensitivity compared to those using Lipidure, Nafion, and poly(ethylene glycol) polymers112. Moreover, the use of a chitosan film as a protective layer can mitigate the effects of proteins, thus extending the lifetime of the wearable electrode114.
Managing fluctuations in substrate availability and controlling fluids
In real-life scenarios, the secretion of biofluids can vary in both volume and concentration of metabolites. Passive sweat sampling platforms may experience saturation or dilution over time, making them unsuitable for prolonged operation. Utilizing fluidic configurations is a strategy to address substrate depletion effectively.
Designing fluidics using wicking materials115 or structured microchannels116 enables the sequential tracking of target flow from the sweat gland through the detection zone, eventually reaching the outlet. The fluctuating availability of metabolites is particularly critical for self-powered biosensors. For instance, a nanofiber-based microfluidic analysis system exemplifies this approach117. This system is comprised of hydrophilic and porous polyimide/sodium dodecyl sulfate nanofiber films. This architectural design mitigates the potential for chemical-induced skin damage caused by the release of soluble chemical reagents from microfluidic networks with hollow structures. Moreover, the utilization of this microfluidic system based on nanofibers assures complete sweat penetration into the sensing chambers, while simultaneously keeping the sensing arrays against impurities released by the skin. Additionally, an osmotic sweat extraction hydrogel coupled with a paper microfluidic channel was demonstrated to facilitate sweat transport, addressing the challenge of fuel availability (Fig. 5a)118. The collected sample undergoes processing and analysis via a paper microfluidic channel, featuring an extended site for evaporation at the end, allowing for long-term sensing from fresh sweat. Another fluidics platform for sample management is a stretchable cotton thread-embedded microfluidic system, enabling a constant absorption rate under various mechanical deformations (Fig. 5b)119. Additionally, silicone elastomer-based microfluidic channels can be constructed and assembled on the sensing interface for high-throughput sweat sampling120,121,122,123,124. Furthermore, studying fluid dynamics simulation is essential for understanding the flow behavior of the microfluidic system.
a Wearable sensor featuring an osmotic hydrogel and a paper microfluidic channel for sweat extraction and management. Adapted with permission from ref. 118. © 2022, American Chemical Society. b Stretchable microfluidic-integrated glucose biofuel cell-based sensor utilizing a cotton thread-embedded microfluidic device. Adapted with permission from ref. 119. © 2022, Wiley‐VCH GmbH. c Air-breathing biocathode employing a superhydrophobic-based design to create an air-solid-liquid triphase for wearable lactate biofuel cells. Adapted with permission from ref. 145. © 2023, Elsevier B.V. d Breathable and woven electrode designed for lactate biofuel cells. Adapted with permission from ref. 146. © 2023, Elsevier Ltd. e Polychlorotrifluoroethylene (PCTFE) based oxygen-rich cathode serving as an internal oxygen supply. Adapted with permission from ref. 147. © 2018, Elsevier B.V.
Microfluidic valve technologies are important for precisely manipulating fluid flow within wearable systems125,126,127,128. This capability is essential for directing fluids to specific areas or chambers within a microfluidic device, facilitating controlled flows and reactions129. Important considerations in designing microfluidic valves include valve type, materials compatibility, actuation method, flow rate, and microchannel dimensions. Factors influencing flow rate modulation, such as channel geometry, fluid viscosity, and system design, must be carefully addressed. Flow rate variations within microfluidic systems can affect fluid handling efficiency, mixing, and reaction kinetics. Accurate mixing is crucial for achieving uniform reactions and reliable assay results. Challenges in achieving precise control include fluid dynamics constraints, valve reliability, and the complexity of system integration. Overcoming these challenges requires interdisciplinary approaches and optimization strategies to ensure accurate fluid handling in microfluidic applications.
One useful example is the utilization of capillary bursting valves for passive sweat management in on-body detection, facilitating the analysis of multiple analytes in sweat such as chloride, glucose, lactate, pH, temperature, urea, creatinine, sweat volume, and sweat rate123,124. Additionally, active biofluid management can be achieved using a programmable microfluidic valving system capable of sampling biofluids chronologically, routing them, and compartmentalizing them for biomarker analysis. One such example involves fabricating a programmable microfluidic valving system for on-skin biofluid management and contextual biomarker analysis130. In this example, electronically programmable microfluidic valving is achieved through microheater-controlled thermo-responsive poly(N-isopropylacrylamide) (PNIPAM) hydrogel valves. The thermo-responsive PNIPAM-based hydrogel undergoes expansion or contraction in response to temperature changes above or below its lower critical solution temperature (LCST), induced by activating or deactivating the microheater. When the hydrogel’s temperature is below its LCST, dominant hydrogen-bonding interactions with water molecules lead to hydration and structural expansion. Conversely, when the temperature exceeds the LCST, weakened hydrogen bonds and dominant hydrophobic interactions cause water release and hydrogel shrinkage upon heating the valve area. This active biofluid control strategy addresses challenges posed by flow rate variability on electrochemical sensor response.
An alternative approach to designing a microfluidic patch that allows users to initiate the timing of analysis was achieved through the development of finger-actuated pumps and valves for sweat sensing of multiple analytes131. The integration of finger-actuated pumps, valves, and sensing chambers in this device enables on-demand and electronics-free monitoring of various sweat analytes. To facilitate on-demand assessment of sweat analytes, a mechanism was designed with a system that allows the user to pull a thin tab to initiate the pump, enabling the sample to flow through the channel towards the analysis chamber.
A dual-valved microfluidic device represents another example of programmed time-control sweat collection132. The integration of passive and active valves on soft microfluidics has facilitated precise fluid control. Passive valves establish automatically controlled microfluidic pathways, while active valves, which require external actuation or triggering to open or close, operate on a specific time schedule to isolate captured sweat in a chamber from incoming sweat. Passive capillary burst valves integrated into the soft microfluidic channel were utilized to collect sweat in separate chambers in a sequential manner. Importantly, a thermal expansion valve, employing heat-expandable microspheres and polydimethylsiloxane (PDMS), was used to programmatically control sweat accumulation. The instantaneous activation of the thermal expansion valve hinders the microfluidic pathway, minimizing sample contamination and molecular diffusion. Studies involving humans demonstrated that the sweat analyte data obtained from this time-programmed sweat collection device are more comprehensive than those obtained from conventional methods, which lack specific data on temporal variations in sweat composition, such as volume, pH, lactate, and cortisol.
The typically low concentration of O2 in most solutions, often less than 1 mM133,134, poses another challenge for on-body enzymatic reactions. For example, glucose oxidase-based amperometric sensors may suffer from reduced performance due to insufficient O2 availability. GOx, immobilized on typical glucose biosensors, relies on O2 as a co-substrate for its enzymatic reaction. This limitation can lead to decreased sensitivity and errors in GOx amperometric sensors, as well as limited power output in EBFCs due to the low amount of O2 supplied to the cathode during the O2 reduction reaction.
To tackle this challenge, one strategy involves the development of air-breathing materials to enhance the solubility and mass transfer of O2. A superhydrophobic electrode facilitates an efficient air-liquid-solid three-phase interface, enhancing O2 diffusion compared to a solid-liquid two-phase interface. This is due to the higher diffusion coefficient of O2 in air than in liquid135. The superhydrophobic substrate is achieved through a combination of composite surface structures and low surface energy materials, trapping air pockets beneath the electrode when immersed in solution136. Breathable electrodes should possess high porosity, gas permeability, lightweight, biocompatibility, and flexibility, enabling easy access of oxygen from the air to the sensing interface. Various structures, such as nanomesh137, porous138, textile139, and nanofiber network140, have been incorporated to create air-breathable electrodes. Common materials used include fabric141, nanofiber-based polytetrafluoroethylene/silver nanowires/silk fibroin nanofibers142, polyamide nanofibers with silver nanowires140, fibrous polyimide143, fibrous PDMS144. An example of this approach is the fabrication of a superhydrophobic base electrode, which is utilized to create an “air-sac” layer at the air-solid-liquid triphase interface, thereby enabling air-breathing capability. This process effectively increases the accessibility of O2 from the air to the liquid phase (Fig. 5c)145. The “air-sac” layer exhibited an approximately 120% increase in the rate of O2 reduction. Another example of enhancing breathability in devices is demonstrated by a woven hybrid energy harvester (Fig. 5d)146.
Another approach to address this oxygen limitation uses an oxygen-rich cathode binder material based on polychlorotrifluoroethylene (PCTFE). This material can provide an internal O2 supply for the EBFC reduction reaction even under O2-deficit conditions (Fig. 5e)147, owing to the high O2 solubility in the PCTFE binder. The use of an O2-rich cathode can improve bioenergy conversion systems when O2 levels are low or fluctuating. This principle applies to both traditional enzyme-based amperometric sensors148 and EBFCs147.
Considering biocompatible and ‘green’ materials
In wearable technology, prioritizing biocompatible and environmentally friendly “green” materials is essential for user safety and environmental sustainability. Examples include incorporating egg white proteins (Fig. 6a)149 and silk fibroin (SF)150 to develop sustainable electrodes for bioelectronics, utilizing their 3D network structure for enzyme entrapment and biodegradability. Additionally, food-based edible EBFCs, which utilize plant and fruit extracts alongside edible charcoal, offer further “green” alternatives151.
a A glucose amperometric biosensor and a bioanode for a BFC and self-powered biosensor using redox-mediated AuNPs, glucose oxidase, and egg white proteins. Adapted under the terms and conditions of CC-BY 4.0149, © 2023, Natcha Rasitanon et al., published by MDPI. b A bio-active porous enzymatic nanofiber membrane composed of silk fibroin nanofibrils (SFNFs) and enzymes for enzymatic biosensor with long-term stability. Adapted with permission from ref. 150. © 2021, Wiley‐VCH GmbH.
SF is a material to form a scaffold to anchor enzymes152. Additionally, silk proteins offer a biodegradable character. A fibroin substrate derived from the silk fibroin was used in glucose biosensors, degrading after 10 days153. A bio-active porous enzymatic nanofiber (PEN) membrane based on silk fibroin nanofibrils (SFNFs) effectively retains enzymes in 3D functional scaffolds (Fig. 6b)150. Glutaraldehyde was added to cross-link SFNFs, transforming the protein bulk structures into three-dimensional skeletons and enabling the formation of the enzymatic membrane. This process provides a large surface area for biochemical reactions when the analyte reaches the immobilized enzyme membrane. These features reduce enzyme escape, promote enzyme-analyte reactions, and ensure stability. In addition, there is reported that GOx entrapped in SF can remain stable for up to 10 months154. Platinum nanoparticles-graphene (Pt-graphene) nanocomposite attaches the PEN membrane, facilitating electron transfer between enzymes and electrodes, resulting in long-term stability and effective glucose and lactate sensing for a duration of up to 25 hours. Pt-graphene film also enhances catalytic activity against H2O2 and reduces its potential to oxidize H2O2. Furthermore, Pt-G film interacts with enzymes and SFNFs, reducing enzyme loss during prolonged sensor operation.
Accessing biofluids
Sweat analysis provides convenient access to biofluids from the human body; however, exploring alternative biofluids such as interstitial fluid (ISF) offers opportunities to gather additional valuable information. In addition to passive sweat sampling, using ISF, which exists in extracellular spaces and directly emanates from blood vessels, holds promise for biomedical diagnosis155. ISF offers advantages such as abundance, minimal bio-interference, and high clinical relevance. Given the wealth of information within ISF, on-skin biosensors have been developed for minimally invasive analysis to extract data from ISF156. Key methods used to access ISF include microneedles157 and iontophoresis158.
Microneedles are employed for ISF extraction by penetrating the skin and can be either solid or hydrogel-based materials159,160. Typically, these microneedles are shaped as small pyramids or cones with sharp tips161. The material properties of microneedle electrodes influence the performance and stability of biosensors integrated with microneedle arrays. Conductive polymers, such as poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonate) (PEDOT: PSS)162, offer high conductivity and compatibility with enzyme immobilization, providing a miniaturized platform for enzyme attachment and signal transduction. Gold electrodes, owing to their conductivity, biocompatibility, and stability, are also viable options163. Gold surfaces can be functionalized with self-assembled monolayers (SAMs) to facilitate enzyme binding. Challenges in achieving effective electron communication between enzymes and microneedle electrodes include the distance between the enzyme active site and the microneedle surface, electron transfer rate, loss of enzyme activity, and biofouling. Additionally, designing microneedle geometries for on-body biosensor applications targeting ISF sampling involves several key considerations for effective performance, including needle length and diameter, material type, microneedle tip design, biocompatibility, skin interaction, and user-friendliness. The length of microneedles should be sufficient to penetrate the outer layer of the skin (epidermis) and reach the dermal layer where ISF resides, typically ranging from 150 to 1500 μm164,165. The diameter of microneedles should be small enough to minimize pain and tissue damage but large enough to allow access to biofluid. The geometry of microneedle tips significantly influences skin penetration efficiency and pain reduction, with shapes such as conical162, pyramidal166,167, or beveled168 designs. It is crucial to utilize biocompatible materials and surface coatings to minimize the risks of skin irritation, inflammation, or infection. Conical or tapered tip geometries are preferred for facilitating skin penetration and reducing discomfort, with tip angles typically ranging from 15° to 60°; sharper angles generally require less insertion force169. Microneedle diameters typically range from 30 μm to 200 μm, with larger diameters allowing for higher fluid flow rates and sensor integration, albeit potentially causing tissue damage and discomfort170,171,172.
An example of fabricating a microneedle-based electrochemical biosensor for glucose and lactic acid detection involves using a hydrogel that can change its form at room temperature in the presence of a photoinitiator and light173. Photolithography was employed to produce polymeric microneedles, which were constructed from photocurable materials including vinylferrocene, enzymes, polyethylene glycol diacrylate (PEGDA), and a photoinitiator. Vinylferrocene, with a redox potential around 0.2 to 0.3 V, served as a redox mediator. The biosensing principle relied on the oxidation of analytes (glucose or lactic acid) catalyzed by enzymes (GOx or lactate oxidase), mediated by the redox mediator molecule, which carried produced electrons from the redox center of the enzyme to the electrode surface. Glucose and lactate levels were determined using amperometry at an applied potential of 0.30 V. These biosensors hold promise for continuously monitoring glucose levels in diabetic patients’ blood or detecting lactic acid levels in athletes.
Another example involves the use of composites of gold and multi-walled carbon nanotubes (MWCNTs) to fabricate a microneedle for monitoring lactate and glucose174. The microneedle array base structures were fabricated using polycarbonate, with pyramid dimensions of 0.06 cm base and 0.1 cm height, covering a 4 × 4 array area of 0.2 cm2. Subsequently, the microneedle array was coated with gold to create the conductive electrode. The working electrode was then coated with Au-MWCNTs to enhance its electrochemical characteristics. Polymethylene blue was layered onto the electrode surface to serve as a redox mediator. FAD-glucose dehydrogenase (FADGDH) and lactate oxidase were immobilized using a drop-casting procedure to obtain the biorecognition layers for detecting glucose and lactate concentrations, which could be determined through amperometric analysis at an applied potential of 0.15 V. This device effectively monitored lactate and glucose concentrations in an artificial skin model.
In addition to using needles to pierce the skin, iontophoresis offers an alternative method for accessing ISF in wearable devices175. This technique employs an electric voltage with a small current to drive the movement of charged ions, inducing an electroosmotic flow that facilitates the extraction of substances from the skin176. The use of electric current enhances the rate of substance transport beyond their passive permeabilities, mainly facilitated by electromigration and electroosmosis mechanisms. Both iontophoresis and reverse iontophoresis use controlled electrical currents to transport ions, molecules, and drugs across biological membranes, such as the skin, in a non-invasive manner. In iontophoresis, a small current drives charged ions or drugs through the skin, overcoming its natural barrier properties for transdermal delivery177. Conversely, reverse iontophoresis reverses the ion movement direction to extract molecules from the body through the skin178. The contribution of each mechanism depends on the physicochemical properties of the substances involved.
The choice of electrode material is crucial in both iontophoresis and reverse iontophoresis applications, directly impacting the efficiency and safety of the device. Silver-based or carbon-based electrodes are commonly used in iontophoresis179,180. In reverse iontophoresis, the electrical current typically ranges from about 0.1 to 4 mA, with the specific current level depending on device design, target molecules, and skin properties181,182. The size of iontophoretic electrodes is similar to sensing electrodes, typically in the millimeter range. Materials commonly used for iontophoretic electrodes include silver-based183, carbon-based184 materials, stainless steel185, and certain polymeric materials186. The properties of these materials directly impact the efficiency and safety of iontophoretic applications. For example, high electrical conductivity ensures efficient ion transport and reliable current distribution across the skin, enhancing effectiveness for drug delivery or sample collection. Electrode materials should also be biocompatible to minimize the risk of skin irritation or allergic reactions during prolonged use.
In addition to the electrode material, a critical parameter governing iontophoretic systems includes the magnitude of electrical current applied, determining the rate and extent of ion or molecule transport across skin. Typically, low currents ranging from 0.1 mA to a few mA are used for safe and controlled delivery or extraction of substances182,187. The duration of the current application also influences the amount of substance delivered or extracted. While longer application times can enhance molecule transport, care must be taken to avoid excessive exposure that could lead to adverse effects.
Examples of reverse iontophoretic ISF extraction in biosensing systems were developed using a pair of screen-printed Ag/AgCl electrodes158,188. To extract ISF, a current density of about 0.2 to 0.3 mA cm−2 was applied to the skin through cathode and anode iontophoresis electrodes for 5 min. These wearable biosensor platforms demonstrated potential for alcohol and glucose monitoring.
Advancements in material and system designs are driving improvements in the effectiveness and safety of iontophoretic techniques, particularly in drug delivery and noninvasive biomarker sampling from biofluids extracted from the skin. Designing effective iontophoretic electrodes involves addressing challenges such as optimizing electrode size for targeted sampling areas, ensuring uniform current distribution, maximizing skin contact and comfort, and enhancing electrode biocompatibility. However, employing the iontophoresis approach presents challenges, such as limitations in fluid extraction volume and the impracticality of prolonged extraction times for biosensing applications. Researchers are tackling these challenges through approaches such as the use of electrode materials such as carbon-based materials and conductive polymers, employing microfabrication techniques to create customized electrode shapes, developing electrode arrays for improved current distribution, integrating real-time monitoring systems for feedback control, and incorporating human factors and user-centered design principles. These endeavors aim to enhance the effectiveness, reliability, and safety of iontophoretic techniques in applications such as drug delivery and noninvasive biomarker sampling, ultimately improving user outcomes and the usability of wearable devices.
Powering biosensing systems with enzyme-based energy devices
EBFCs function as power sources by converting biochemicals from human biofluids into electricity. They offer the advantage of ensuring a continuous energy supply for wearable electronic devices189. Compared with conventional energy sources such as batteries, EBFCs have garnered significant attention due to their high efficiency and environmental friendliness. These bioenergy devices provide a power solution for small-scale, on-demand electronics190. EBFCs typically yield power ranging from μW cm−2 to mW cm−2, making them well-suited for wearable sensors. Their portability and eco-friendly operation make them ideal for supporting wearable biomedical and miniaturized electronic devices. Harnessing EBFCs addresses energy needs in sensors and small personal bioelectronics, offering sustainable solutions while reducing environmental impact and decreasing reliance on traditional energy sources191.
While progress has been made in developing a biobattery-based patch that can operate on the skin, achieving shape conformability on the skin and rapid on-site activation remains a challenge. During storage, enzymatic batteries should be dried to preserve the activity of enzymes. Before use, they must be moistened to initiate enzymatic reactions on the electrodes. An example is a water-activated electric skin patch capable of producing transdermal current promptly, which integrates a built-in flexible glucose/O2 biobattery capable of generating current192. The main components of this integrated patch include a flexible biobattery and water-absorbent sponge tanks for storing the buffer electrolyte and fuel. Dehydrated fuel and electrolytes can be stored in the sponge, which can also rapidly absorb water and retain the resulting solution. Rapid activation of the patch components through the addition of water deep within the patch is achieved by employing a flexible sponge saturated with buffer electrolyte and glucose (the primary source of biofuel). In this device, CNT-modified carbon fabric was used as the electrode substrate to enhance surface area and conductivity. Glucose dehydrogenase was immobilized on the electrode to fabricate the anode, while iron(II) phthalocyanine was used to construct the cathode to catalyze the O2 reduction reaction. The wet cotton sheet containing the electrolyte solution exhibited a current of approximately 80 μA, while human skin displayed a current of approximately 10 μA. A maximum power density of 6 W cm−2 was achieved by the biobattery on a cotton sheet saturated with a buffer solution containing 200 mM glucose at 0.2 V. A stable patch current of about 10 μA was observed for over 12 h in the soft spongy container containing 200 mM glucose biofuel and electrolyte, confirming the stability of the output current during prolonged measurements.
Utilizing the principle of EBFCs could be beneficial for fabricating a wearable sensing device operated entirely on self-generated power, eliminating the requirement for an extra power source193. EBFCs, which convert glucose in urine into electrical energy, were utilized to power the diaper system. Carbon nanotubes/gold nanoparticles (CNTs/AuNPs) hybrids were employed on the glucose oxidase-based bioanode to enhance the output performance of EBFCs. Enzyme immobilization was achieved using glutaraldehyde to cross-link GOx with CNTs/AuNPs hybrids before coating on the electrode. MnO2-CNTs were used as the cathode to accept electrons from the bioanode. The hybrid material composed of CNTs/AuNPs is a promising candidate for facilitating efficient electron transfer between the electrode surface and the immobilized enzyme due to its small size and high electronic conductivity. When the EBFC operates in a 5 mM glucose solution, its power density can reach 220 μW cm−2. The EBFC was integrated into the diaper and connected to a power management system equipped with a capacitor for energy storage. The power management system, including a capacitor for energy storage, was linked to the EBFC integrated into the diaper. The light-emitting diode (LED) flashed in response to the stored energy, which was subsequently used to display the observable signal. The power generated by the EBFC regulated the charging rate of the capacitor, which was reflected in the frequency of LED flickering. As a result, the flashing LED provided a direct indication of the glucose concentration in the urine.
Several biomolecules other than glucose can also serve as energetic sources for the biofuel system. One notably important biofuel is lactate. An example of this was demonstrated in a hybrid EBFC, which was designed to supply electricity for powering wearable devices194. These EBFCs, utilizing poly(vinyl alcohol)-based (PVA-based) hydrogel electrolytes, were integrated with lactate oxidase/tetrathiafulvalene-MWCNTs/reduced graphene oxide/porous Ni substrate bioanodes, and Ag/Ag2O cathodes. The hydrogel exhibited significant capacity for absorbing lactate-containing PBS electrolyte solutions. The integrated PVA-based hybrid biofuel cell demonstrated a maximum power density of ~ 129 μW cm−2 and an open circuit voltage of 0.50 V when operating in a 20 mM lactate solution. When human sweat was utilized as the biofuel, the hybrid biofuel cell had the potential to achieve a maximum power density of ~ 85 μW cm−2. Furthermore, when coupled with a DC-DC converter, the PVA-based hybrid biofuel cells exhibit the capability to supply power to a watch.
To realize integrated wearable systems, researchers are striving to create standalone devices by merging various functional platforms. These platforms should encompass not only EBFCs but also extend to other biosensors and energy storage units (e.g., supercapacitors). An example includes the demonstration of hybrid energy devices that integrate supercapacitors with EBFCs195. These devices incorporate anodes and cathodes based on buckypaper@polydimethylsiloxane electrodes that incorporate enzymes reacting with respective fuels and mediators facilitating the transfer and storage of electrons. On the anode side, FADGDH, an enzyme facilitating glucose oxidation, was employed, accompanied by the utilization of 1,10-phenanthroline-5,6-dione as a mediator. On the cathode, bilirubin oxidase, an enzyme facilitating oxygen reduction, was utilized, with 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) serving as the mediator. The hybrid energy devices exhibited a power output of 0.93 mW cm−2 and a capacitive storage capacity of 307 mF cm−2. The performance of this hybrid device, using electrodes comprising both enzymes and mediators, was attributed to their self-charging capability. In addition, after two charge–discharge cycles in the presence of a glucose solution, the open circuit voltage (OCV) remained stable for up to 800 s at 0.675 V, even in the absence of fuel, enabling the discharge of the hybrid energy device. It was confirmed that the integrated energy devices exhibited performance characteristics of both a fuel cell and a supercapacitor.
Advancing on-body analysis settings for real sweat analysis
Researchers have extensively studied various biomarkers in real biofluids, with glucose and lactic acid emerging as popular choices. The concentration ranges of glucose and lactate from electrochemical sensors between 2016 and 2024 are summarized in Fig. 7. Glucose and lactate levels in real sweat were found to range from approximately 2.0 × 10−5 to 1.5 × 10−3 M and 2.0 × 10−4 to 6.8 × 10−2 M, respectively. These values vary depending on the human subject and testing conditions, influenced by factors e.g., physiological conditions and hydration levels196,197. Physical activity, particularly intense or prolonged exertion, can elevate sweat lactate levels198. Additionally, stress, diet, and overall health can contribute to metabolic variations. Lactate production increases during anaerobic metabolism in exercise, leading to a high lactate concentration in sweat. Dehydration may also result in elevated lactate levels199. During intense exercise, e.g., field track running, treadmill running, and ergometer cycling, lactate concentrations can reach up to 68 mM200. Notably, health issues (e.g., diabetes) can alter sweat compositions, impacting glucose levels. In sensor development, it is crucial to account for both lower and upper limits of analyte concentration, ensuring the sensor’s capability to detect trace levels while also accommodating high concentrations. Achieving high sensitivity is essential to detect analytes at trace levels, while maintaining the sensor’s ability to detect high concentrations without saturation effects ensures its practical utility across a wide range of expected situations.
The reported concentration ranges of a glucose and b lactate in real sweat collected from electrochemical sensors. Data sourced from refs. 60,61,71,79,85,105,117,118,120,150,198,200,201,202,203,205,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259 for glucose and lactate.
We first highlight representative cases displaying the two lowest concentration levels of sweat glucose and lactate, as depicted in the bottom positions on the bar charts within the literature’s data. These enzyme-based biosensors can determine sweat glucose at concentrations as low as 20 and 30 µM, achieved through different materials: one utilizing an enzyme-based Au electrode with CNTs and Prussian blue201, and the other employing a laser-induced porous graphene electrode with platinum nanoparticles (PtNPs)85. The GOx was entrapped in chitosan and CNT matrix for Au electrode and Prussian blue. The Prussian blue was used as a mediator to catalyze H2O2 reduction. On the other hand, the GOx immobilized on laser-induced graphene electrode by using coupling agent (EDC/NHS) to activate the carboxylate groups on the electrode surface. This electrode can directly detect H2O2 with the aid of PtNPs. The sensor’s capabilities are attributed to the following features; for instance, the Au electrode demonstrates electrocatalytic activity and conductivity, thereby enhancing electron transfer. Additionally, the PtNPs-modified porous graphene electrode offers a surface for enzyme immobilization and facilitates efficient communication between the electrode and enzyme active sites.
Regarding lactate detection, textile-based self-powered sensors achieved low concentrations of around 0.2–1.0 mM. One sensor utilized stretchable CNT ink with a 1,4-naphthoquinone mediator bioanode and silver oxide/silver (Ag2O/Ag) cathode79, while the other employed flexible screen-printed Prussian blue-based ink with a graphene and enzyme mixture layer118. These platforms detected such low lactate concentrations due to mediators (1,4-naphthoquinone and Prussian blue) and nanomaterial-based inks (stretchable CNT and Prussian blue-based carbon materials), along with enzyme immobilization techniques such as Nafion and chitosan coatings. We also highlight examples exhibiting the two highest concentration levels of sweat glucose and lactate, represented by the rightmost positions on the bar chart. The high glucose concentrations observed in sweat were 0.31 and 1.5 mM, detected using screen-printed platinum-decorated graphite bioelectrode202 and Prussian blue-graphite bioelectrode203. These bioelectrodes were prepared by coating GOx with Nafion, and the detections relied on catalytic reaction of H2O2 on the platinum and Prussian blue surface. The sensor’s ability to cover a wide concentration range was attributed to the high surface area and electrocatalytic activity of the supporting materials for enzyme immobilization.
The high concentration of lactate detected in real sweat was achieved by enzymatic sensors using ZnO nanoflakes coated on Au electrodes61, and surface-modified, screen-printed carbon electrodes200, reaching concentrations of 50.7 mM and 68 mM, respectively. Enzyme immobilization was accomplished through electrostatic physical adsorption and covalent bonding using EDC–NHS, respectively. Lactate detection in the former relied on H2O2 oxidation, while in the latter, it relied on changes in charge transfer resistance on the enzyme electrodes.
Multiplex biosensing systems and diagnostic-therapeutic integration
Wearable technology has enabled the effective study of predominant biofluid analytes such as glucose, lactate, K+, and Na+. Innovations in wearable devices have focused on enhancing reliability in on-body analysis settings. In biomedical applications, key challenges related to on-body settings need to be considered. Ensuring the practicality of wearable biosensors and bioelectronics in real analysis settings is essential. The variability in sample matrix among individuals can affect sensor accuracy, requiring thorough validation. Challenges in on-body settings include managing unstable signals and noise induced by human movement, as well as ensuring the mechanical stability of wearable devices to closely adhere to body movements for practicality. Finding suitable device placement locations that remain steady and comfortable for users while providing access to valuable information from biofluids poses another significant challenge. Advancements in wearable biosensors and bioelectronics have addressed many limitations of traditional on-body analysis settings. Lightweight, miniaturized, and flexible platforms enable seamless integration with daily activities. Additionally, self-powered sensing platforms eliminate the need for bulky batteries, ensuring long-term analysis capability while reducing device size. Following the development of materials and integration with relevant enzymes for building desired sensors, some representative ideas for integrating devices in on-body studies are briefly highlighted.
Introducing a wearable chemical-electrophysiological hybrid biosensing system highlights its capability for simultaneous real-time monitoring of biochemical and electrophysiological signals, showcasing its potential for personalized diagnostic applications. Another example of on-body healthcare diagnostic settings was seen with the development of a wearable chemical-electrophysiological hybrid biosensing system for real-time health and fitness monitoring204. This skin-worn wearable hybrid sensing system offers simultaneous real-time monitoring of a biochemical signal (lactate) and an electrophysiological signal (electrocardiogram). Additionally, an integrated wearable sensor array was developed for multiplexed perspiration analysis, covering glucose, lactate, K+, Na+, and skin temperature201. The wearable sensor was worn on various body parts, including the forehead, wrists, and arms, and successfully applied for on-body analysis. This platform enables multiplexed perspiration analysis in personalized diagnostic and physiological monitoring applications.
The integration of enzymatic biosensors with physical sensors, such as electrocardiogram (ECG), electromyogram (EMG), and electroencephalogram (EEG), represents another approach with significant implications for healthcare. This synergistic fusion enables the simultaneous measurement of biochemical markers alongside physiological signals, offering a comprehensive understanding of health status. One representative system hybridizes epidermal sensors to monitor glucose, pH, temperature, ECG, EMG, and EEG (Fig. 8a)205. The patch was fabricated using microelectromechanical systems (MEMS) technology on a thin, flexible polyethylene terephthalate (PET) film to ensure suitable skin contact. MEMS-patterned Au electrodes on PET support were utilized to directly record electrophysiological signals such as ECG, EMG, EEG, and temperature. PtNPs/nanoporous carbon@MXene-based sensors and polyaniline-based sensors were employed for glucose and pH detection, respectively. This wearable device, paired with a miniaturized printed circuit board (PCB), could assess glucose and ECG levels of the wearer by attaching it to the subject’s chest during stationary cycling. Real-time on-body monitoring was demonstrated by attaching the sensor directly to the subject’s chest. Prior to wearing the sensors, all sensors were calibrated, and the subjects’ chests were cleansed with alcohol swabs. Through the integration of pH and temperature sensors, the responses of the biosensing patch for glucose were internally calibrated to account for changes in pH and sweat temperature during on-body perspiration monitoring. This biosensing patch exhibited selectivity against interfering and biofouling effects, attributed to the catalytic properties of PtNPs in reducing the detection potential of H2O2 and the permselective protective layer of Nafion.
a An electronic skin for stress response monitoring. Adapted with permission from ref. 206. © 2024, The Author(s), under exclusive license to Springer Nature Limited. b A biosensing patch for biopotentials and sweat glucose monitoring. Adapted with permission from ref. 205. © 2022, Wiley‐VCH GmbH. c The battery-free and wireless wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery. Adapted with permission from ref. 215. © 2021, Wiley‐VCH GmbH. d Multifunctional conducting polymer-based theragnostic bandage for multiplex sensing and drug delivery capabilities. Adapted under the terms and conditions of CC-BY 4.0216, © 2024, Lingyin Meng et al., published by Springer Nature.
A soft sensor was developed for monitoring stress responses, capable of classifying different stressor types (cold pressor test, virtual reality challenge, intense exercise) and predicting state anxiety levels with high accuracy and reliability using a machine learning pipeline (Fig. 8b)206. The device is capable of evaluating three essential indicators of health—pulse waveform, galvanic skin response, and skin temperature—and monitoring six specific substances found in sweat: glucose, lactate, uric acid, sodium ions, potassium ions, and ammonium. It was affixed to the subject’s wrist for on-body analysis, utilizing a microfluidic module to access sweat. The incorporation of a miniaturized iontophoresis component enabled the self-sustaining stimulation of sweat production while at rest, eliminating the necessity for strenuous physical activity. Various on-body locations such as the forehead, forearm, chest, and shoulder could be considered for attachment, ensuring comfort and minimal movement for reliable sample collection. Enzymatic sensors based on Prussian blue with a nickel hexacyanoferrate coating exhibited the ability to eliminate interfering effects, operating at a detection potential of 0 V. This biosensor provided long-term sweat biomarker analysis for over ~ 4 days in untreated human sweat samples, attributed to the catalytically inactive nickel hexacyanoferrate forming a stabilized solid solution composite, thereby protecting the Prussian blue.
Ultimately, wearable devices aim not only to detect biochemical information for diagnostic purposes but also to utilize it for dynamically adjusting treatment regimens. The objective is to unlock the potential for optimizing therapeutic interventions by providing timely drug delivery tailored to individual needs. One of the most beneficial integrations is incorporating on-body sensors for wound monitoring and drug delivery applications. Therefore, researchers aim to further design integrated systems for real-time, noninvasive monitoring of biochemical markers, enabling the assessment of wound healing progress and early detection of complications.
Effective wound management is an essential component of healthcare. Effective management of individual wounds is essential for facilitating wound healing, frequently necessitating the utilization of wound bandages or dressings. Various physiological parameters present in wound exudate, such as temperature, pH, uric acid, glucose, and total protein, can be monitored using multiplex sensors and biosensors207,208,209. Continuous and prolonged monitoring of these biomarkers in real-time can offer valuable insights into the healing progress and the conditions of the wound environment, specifically regarding the potential for infection.
For instance, the normal pH of skin or healing wounds typically falls between 5.5 and 6.5, whereas chronic or infected wounds with a high bacterial load often exhibit a basic pH above 7.3210,211. Furthermore, there is a significant correlation between the severity of wounds and the concentration of uric acid in wound exudate, which is linked to the colonization of bacteria such as Staphylococcus aureus or Pseudomonas aeruginosa and the generation of reactive superoxide radicals212,213. Consequently, the ability to determine the difference between the severity and infection of a wound is facilitated by the simultaneous detection of pH and uric acid concentrations. Furthermore, in accordance with the paradigm of timely and individualized treatment, investigations need to integrate a therapeutic system into bandages for wounds.
An example illustrating the design of a wearable bioelectronic system demonstrated its efficacy in monitoring the physiological conditions of wounds through multimodal wound biomarker analysis, while concurrently facilitating combination therapy via electro-responsive controlled drug delivery214. The system comprised sensors for glucose, lactate, uric acid, temperature, pH, and NH4+. The wearable patch collected multiplexed biomarker data, which provided insights into temporal and spatial variations in the wound microenvironment and the inflammatory condition of the infected wound at various stages of the healing process. In addition, the integration of electrical stimulation on the wearable technology together with electrically modulated antibiotic delivery significantly expedited the closure of chronic wounds.
The development of fully integrated wound-dressing-based devices represents a significant advancement in wearable platforms, enabling continuous wound monitoring alongside on-demand drug delivery. Extensive research efforts have focused on integrating responsive drug delivery systems into smart wound dressings, improving drug dosage precision and enabling tailored therapy for enhanced patient outcomes. One development reported an integrated, battery-free, and wireless smart wound dressing for wound infection monitoring and drug delivery (Fig. 8c)215. The wearable electronics were fabricated based on a polyimide substrate to achieve a flexible platform. The multiplex sensor was developed for the simultaneous detection of temperature, pH, and uric acid to assess wound conditions. The uric acid sensor was based on a nonenzymatic sensor using reductive graphene oxide and gold nanoparticles on the electrode. A polyaniline-coated AuNPs sensor was adapted for pH measurement, while temperature was monitored using a temperature sensor chip. In addition to the sensing module, the drug delivery electrode contained cefazolin antibiotics coated in a polypyrrole (PPy) layer, providing on-demand infection treatment through electrically controlled delivery. The cefazolin antibiotic was utilized to treat wounds infected by Staphylococcus aureus. This wound dressing was applied to infected wounds on rats. The decision criteria for initiating drug delivery were based on temperature, pH, and uric acid concentration, serving as indicators signaling the need for drug administration to the subject’s skin. The determination of drug delivery initiation thresholds relied on precedent clinical investigations and pharmacokinetic evaluations, with thresholds set at approximately 35 °C, 8.3, and 358 µM for temperature, pH, and uric acid, respectively. Additionally, observations of uninfected wounds revealed lower values for temperature (approximately 32 °C), pH (approximately 7.6), and uric acid (approximately 269 µM), falling below the established cutoff levels. These settings ensured timely and precise delivery of the therapeutic agent, optimizing its efficacy while minimizing the risk of under- or over-dosing for the user. By integrating these decision criteria into the drug delivery system, it could autonomously respond to the user’s physiological needs, providing personalized and targeted therapy safely and efficiently. The wound of the rat subject was treated with electronically controlled drug delivery by applying a potential of −0.5 V for 90 s. In this process, the PPy film was reduced, releasing the negatively charged molecules of the doped drug into the wound exudate under electrical repulsion and diffusional force. A decrease in the levels of temperature, pH, and uric acid was observed after treatment, indicating that wounds treated with medications showed improved recovery. By capitalizing on the advantages afforded by flexible electronics and wireless communication, this system represents a closed-loop biomedical system that integrates therapeutic interventions with diagnosis.
Another conducting polymer-based array with multiplex sensing and drug delivery capabilities was developed (Fig. 8d)216. This array featured a multiplex sensor and drug delivery system based on different functionalities of conducting polymers, namely, polyaniline, poly(3,4-ethylenedioxythiophene) (PEDOT), and PPy, enabling simultaneous pH and uric acid detection and drug release. A theranostic bandage was fabricated using laser-induced graphene electrode arrays on flexible substrates. The uric acid biosensor was created by immobilizing uricase on a PEDOT: Prussian blue-modified laser-induced graphene electrode. Additionally, alongside the enzymatic electrodes, a pH sensor was incorporated to monitor the alkalinity and acidity conditions of the wound, enhancing overall functionality. The quinolone antibiotic ciprofloxacin was released from the polypyrrole-based drug carrier in response to abnormal pH and uric acid levels detected through electrical stimulation at an applied potential of 0.6 V. The oxidation of the PPy was initiated by positive potential electrical stimulation, which allowed the entrance of anions (e.g., Cl−) and water molecules into the PPy film. This expansion of the film and subsequent extraction of the embedded ciprofloxacin drug occurred as a result. The drug delivery patch successfully inhibited the growth of bacteria, namely E. coli, in vitro, demonstrating potential for wound monitoring and management. The potential of this bandage as a closed-loop drug release system is influenced by future research and clinical validation regarding the correlation between the severity of bacterial infections and levels of uric acid and pH.
Outlook
This review has discussed advancements in wearable enzyme-based biosensors and self-powered biosensors for health monitoring. These technologies utilize advanced functional materials for enzyme conjugation and have undergone improvements in engineering materials and designs. Various strategies have been employed to improve material flexibility, nanostructured design, immobilization strategies, electron transfer efficiency, and biocompatibility. Additionally, efforts have been made to tackle issues related to stability, limited substrate availability, low O2 concentration, biofouling effects, interfering factors, accessing biofluids, and controlling fluids. The discussion also covered enzyme-based energy devices for powering biosensing systems. The concentration ranges of glucose and lactate in real sweat collected from electrochemical sensors have been summarized for reference. Additionally, we have highlighted representative examples that demonstrate the potential of multiplex biosensors in enhancing health assessments by conducting simultaneous detection of related biomarkers and physiological parameters. An example of the integration of on-body sensors facilitating drug release within on-body devices represents a significant advancement. This integration not only enables seamless diagnosis but also extends to therapy, marking a crucial step towards the development of full closed-loop biomedical applications. By bridging the gap between detection and treatment, these research directions hold promise for personalized healthcare interventions tailored to individual needs, ultimately improving overall personalized healthcare.
To ensure the reliability of wearable sensors for healthcare diagnosis, validation is crucial217,218. This involves comparing results with established measurement techniques and evaluating materials and sensor performances, including long-term stability, mechanical stability, and specificity during on-body analysis. Advanced signal processing techniques are also vital for detecting multiple disease-related biomarkers and enriching the comprehensive information profile219,220,221. Collaborative efforts across data science and signal processing are essential for developing efficient algorithms and tools to handle big data from wearable arrays222,223,224. Data scientists can contribute by developing techniques for data calibration and normalization, while signal processing experts can enhance signal quality through algorithm development. Furthermore, integrating multiple sensor types beyond electrochemical sensors allows for a more comprehensive collection of healthcare data225. In particular, the integration of enzymatic colorimetric sensors is noteworthy as it enables visual feedback, empowering individuals to monitor their health status without relying on specialized electronics226,227. Additionally, developing self-sustainable wearable devices for healthcare requires collaboration across materials science, bioengineering, and electronics228. Electronics experts also contribute to miniaturization efforts and efficient electronic and energy interface design. Multiple energy harvesting strategies, such as perovskite solar cells229 and biofuel cells (BFCs)33, can enhance reliability in autonomous wearable biosensors. Besides using extracted enzymes, bioenergy devices can also incorporate microbial-based BFCs as an alternative power source for wearable electronics. For instance, immobilizing the Shewanella oneidensis MR-1 biocatalyst, a well-known exoelectrogen, on the smart textile platform enables the extraction of electrons from microbial metabolism230,231. Moreover, various biorecognition materials, such as DNA, aptamers, and antibodies, can be integrated into these systems alongside enzymes, depending on the desired interaction mechanisms with target biomolecules232,233. Additionally, alternative materials, e.g., enzyme-mimicking inorganic substances, play a crucial role in catalyzing electrochemical reactions234,235. Careful consideration of addressing different challenges is essential. The remaining challenges include addressing efficiency, complexity, and size through hybrid systems, advanced material engineering, and high-performance energy storage. It is also essential to understand biomarker dynamics and their physiological and clinical correlations.
The principles and challenges addressed in developing wearable electrochemical sensors for healthcare diagnosis lay a strong foundation for expanding their applications across various applications. It is possible to expand the applications and shared principles discussed in this review to broaden the impact on other domains in healthcare diagnosis or modern analytical applications. For instance, implantable sensors emerge as a promising avenue in this regard. These implantable sensors, designed for insertion into the body, offer an alternative approach to monitor specific physiological parameters or biomarkers236,237. Typically implanted during surgical procedures, these biodevices can remain in the body for extended periods, facilitating continuous or periodic monitoring of health metrics. Implantable sensors offer advantages such as continuous monitoring, early disease detection, and long-term data collection for chronic health problems. All biosensing modules play pivotal roles in healthcare monitoring and management, providing complementary benefits tailored to specific use cases and individual medical needs. Biosensors operating in vivo, whether on or inside the body, have the potential to improve health outcomes and facilitate personalized health management strategies. These biosensors offer promising opportunities for both individuals and modern healthcare systems, opening up impactful avenues for innovation and advancement. Although sensor design and reusability pose challenges, ongoing interdisciplinary collaboration and technological advancements are driving progress in this field. The growing commercialization of wearable biosensors is anticipated to have a substantial long-term impact on healthcare238,239. Multidisciplinary collaborative efforts among researchers, engineers, clinicians, and interdisciplinary teams are indispensable for advancing our understanding of dynamic results, developing high-performance materials and sensitive detection methods, and improving the quality of wearable biosensors tailored for healthcare diagnosis.
References
Ates, H. C. et al. End-to-end design of wearable sensors. Nat. Rev. Mater. 7, 887–907 (2022).
Nasiri, S. & Khosravani, M. R. Progress and challenges in fabrication of wearable sensors for health monitoring. Sens. Actuators A: Phys. 312, 112105 (2020).
Kim, J. et al. Wearable temporary tattoo sensor for real-time trace metal monitoring in human sweat. Electrochem. Commun. 51, 41–45 (2015).
Wang, Y. et al. Low-cost, μm-thick, tape-free electronic tattoo sensors with minimized motion and sweat artifacts. npj Flex. Electron. 2, 6 (2018).
Parrilla, M., Vanhooydonck, A., Watts, R. & De Wael, K. Wearable wristband-based electrochemical sensor for the detection of phenylalanine in biofluids. Biosens. Bioelectron. 197, 113764 (2022).
Kim, H. et al. A Wearable Wrist Band-Type System for Multimodal Biometrics Integrated with Multispectral Skin Photomatrix and Electrocardiogram Sensors. Sensors 18, https://doi.org/10.3390/s18082738 (2018).
Pal, A. et al. Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages. Biosens. Bioelectron. 117, 696–705 (2018).
Guinovart, T., Valdés-Ramírez, G., Windmiller, J. R., Andrade, F. J. & Wang, J. Bandage-Based Wearable Potentiometric Sensor for Monitoring Wound pH. Electroanalysis 26, 1345–1353 (2014).
Gonçalves, C., Ferreira da Silva, A., Gomes, J. & Simoes, R. Wearable E-Textile Technologies: A Review on Sensors, Actuators and Control Elements. Inventions 3, https://doi.org/10.3390/inventions3010014 (2018).
Islam, G. M. N., Ali, A. & Collie, S. Textile sensors for wearable applications: a comprehensive review. Cellulose 27, 6103–6131 (2020).
Cheraghi Bidsorkhi, H. et al. Wearable Graphene-based smart face mask for Real-Time human respiration monitoring. Mater. Des. 230, 111970 (2023).
Jeerapan, I., Sangsudcha, W. & Phokhonwong, P. Wearable energy devices on mask-based printed electrodes for self-powered glucose biosensors. Sens. Bio-Sens. Res. 38, 100525 (2022).
Sempionatto, J. R. et al. Eyeglasses-based tear biosensing system: Non-invasive detection of alcohol, vitamins and glucose. Biosens. Bioelectron. 137, 161–170 (2019).
Sempionatto, J. R. et al. Eyeglasses based wireless electrolyte and metabolite sensor platform. Lab a Chip 17, 1834–1842 (2017).
Kim, J. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997 (2017).
Seo, H. et al. Smart Contact Lenses as Wearable Ophthalmic Devices for Disease Monitoring and Health Management. Chem. Rev. 123, 11488–11558 (2023).
Moonla, C. et al. Review—Lab-in-a-Mouth and Advanced Point-of-Care Sensing Systems: Detecting Bioinformation from the Oral Cavity and Saliva. ECS Sens. 1, 021603 (2022).
Arakawa, T. et al. A Wearable Cellulose Acetate-Coated Mouthguard Biosensor for In Vivo Salivary Glucose Measurement. Anal. Chem. 92, 12201–12207 (2020).
Abdullah, H., Phairatana, T. & Jeerapan, I. Tackling the challenges of developing microneedle-based electrochemical sensors. Microchimica Acta 189, 440 (2022).
Park, Y.-G., Lee, S. & Park, J.-U. Recent Progress in Wireless Sensors for Wearable Electronics. Sensors 19, https://doi.org/10.3390/s19204353 (2019).
Baghayeri, M., Veisi, H. & Ghanei-Motlagh, M. Amperometric glucose biosensor based on immobilization of glucose oxidase on a magnetic glassy carbon electrode modified with a novel magnetic nanocomposite. Sens. Actuators B: Chem. 249, 321–330 (2017).
Teymourian, H., Barfidokht, A. & Wang, J. Electrochemical glucose sensors in diabetes management: an updated review (2010–2020). Chem. Soc. Rev. 49, 7671–7709 (2020).
Khumngern, S. & Jeerapan, I. Advances in wearable electrochemical antibody-based sensors for cortisol sensing. Anal. Bioanal. Chem. 415, 3863–3877 (2023).
Lee, H.-B., Meeseepong, M., Trung, T. Q., Kim, B.-Y. & Lee, N.-E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 156, 112133 (2020).
Ye, C. et al. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nature Nanotechnology https://doi.org/10.1038/s41565-023-01513-0 (2023).
Singh, N. K., Chung, S., Chang, A.-Y., Wang, J. & Hall, D. A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 227, 115097 (2023).
Yang, C., Denno, M. E., Pyakurel, P. & Venton, B. J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Analytica Chim. Acta 887, 17–37 (2015).
Brainina, K., Stozhko, N., Bukharinova, M. & Vikulova, E. Nanomaterials: Electrochemical Properties and Application in Sensors. 3, https://doi.org/10.1515/psr-2018-8050 (2018).
Chen, A. & Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 42, 5425–5438 (2013).
Ali, A. et al. Recent progress in energy harvesting systems for wearable technology. Energy Strategy Rev. 49, 101124 (2023).
Rong, G., Zheng, Y. & Sawan, M. Energy Solutions for Wearable Sensors: A Review. Sensors 21, https://doi.org/10.3390/s21113806 (2021).
Dahiya, A. S. et al. Review—Energy Autonomous Wearable Sensors for Smart Healthcare: A Review. J. Electrochem. Soc. 167, 037516 (2020).
Bandodkar, A. J. & Wang, J. Wearable Biofuel Cells: A Review. Electroanalysis 28, 1188–1200 (2016).
Wang, L. et al. Enzymatic Biofuel Cell: Opportunities and Intrinsic Challenges in Futuristic Applications. Adv. Energy Sustainability Res. 2, 2100031 (2021).
Jeerapan, I., Sempionatto, J. R. & Wang, J. On-Body Bioelectronics: Wearable Biofuel Cells for Bioenergy Harvesting and Self-Powered Biosensing. Adv. Funct. Mater. 30, 1906243 (2020).
Saha, T. et al. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 123, 7854–7889 (2023).
Verma, D. et al. Internet of things (IoT) in nano-integrated wearable biosensor devices for healthcare applications. Biosens. Bioelectron.: X 11, 100153 (2022).
ul Haque, S., Yasir, M. & Cosnier, S. Recent advancements in the field of flexible/wearable enzyme fuel cells. Biosens. Bioelectron. 214, 114545 (2022).
Khan, A. et al. A review on advanced nanocomposites materials based smart textile biosensor for healthcare monitoring from human sweat. Sens. Actuators A: Phys. 350, 114093 (2023).
Khaleque, M. A. et al. Nanostructured wearable electrochemical and biosensor towards healthcare management: a review. RSC Adv. 13, 22973–22997 (2023).
Wu, H., Zhang, Y., Kjøniksen, A.-L., Zhou, X. & Zhou, X. Wearable Biofuel Cells: Advances from Fabrication to Application. Adv. Funct. Mater. 31, 2103976 (2021).
Wang, J. et al. Flexible and wearable fuel cells: A review of configurations and applications. J. Power Sources 551, 232190 (2022).
Garland, N. T., Kaveti, R. & Bandodkar, A. J. Biofluid-Activated Biofuel Cells, Batteries, and Supercapacitors: A Comprehensive Review. Adv. Mater. 35, 2303197 (2023).
Song, Y., Mukasa, D., Zhang, H. & Gao, W. Self-Powered Wearable Biosensors. Acc. Mater. Res. 2, 184–197 (2021).
Maduraiveeran, G., Sasidharan, M. & Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 103, 113–129 (2018).
Lee, C.-K. & Au-Duong, A.-N. Enzyme Immobilization on Nanoparticles: Recent Applications in Emerging Areas in Bioengineering (ed Ho Nam Chang) 67-80 (Wiley-VCH, 2018). https://doi.org/10.1002/9783527803293.ch4
Arabacı, N., Karaytuğ, T., Demirbas, A., Ocsoy, I. & Katı, A. Nanomaterials for Enzyme Immobilization in Green Synthesis of Nanomaterials for Bioenergy Applications 165-190 (2020). https://doi.org/10.1002/9781119576785.ch7
Ansari, S. A. & Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 30, 512–523 (2012).
Di Bari, C. et al. Fabrication of high surface area graphene electrodes with high performance towards enzymatic oxygen reduction. Electrochim. Acta 191, 500–509 (2016).
Khan, R. et al. Two-Dimensional Nanostructures for Electrochemical Biosensor. Sensors 21, https://doi.org/10.3390/s21103369 (2021).
Lu, X., Zhang, H., Ni, Y., Zhang, Q. & Chen, J. Porous nanosheet-based ZnO microspheres for the construction of direct electrochemical biosensors. Biosens. Bioelectron. 24, 93–98 (2008).
Li, Y., Shi, L., Han, G., Xiao, Y. & Zhou, W. Electrochemical biosensing of carbaryl based on acetylcholinesterase immobilized onto electrochemically inducing porous graphene oxide network. Sens. Actuators B: Chem. 238, 945–953 (2017).
Kucherenko, I. S., Soldatkin, O. O., Kucherenko, D. Y., Soldatkina, O. V. & Dzyadevych, S. V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: a review. Nanoscale Adv. 1, 4560–4577 (2019).
Xu, J., Wang, Y. & Hu, S. Nanocomposites of graphene and graphene oxides: Synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchimica Acta 184, 1–44 (2017).
Newman, J. D. & Setford, S. J. Enzymatic biosensors. Mol. Biotechnol. 32, 249–268 (2006).
Nguyen, H. H., Lee, S. H., Lee, U. J., Fermin, C. D. & Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 12, https://doi.org/10.3390/ma12010121 (2019).
Sassolas, A., Blum, L. J. & Leca-Bouvier, B. D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 30, 489–511 (2012).
Bollella, P. & Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 10, 157–173 (2018).
Li, L. et al. Fully integrated wearable microneedle biosensing platform for wide-range and real-time continuous glucose monitoring. Acta Biomaterialia 175, 199–213 (2024).
Zheng, L., Liu, Y. & Zhang, C. A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat. Sens. Actuators B: Chem. 343, 130131 (2021).
Alam, F. et al. Flexible and Linker-Free Enzymatic Sensors Based on Zinc Oxide Nanoflakes for Noninvasive L-Lactate Sensing in Sweat. IEEE Sens. J. 20, 5102–5109 (2020).
RoyChoudhury, S. et al. Continuous Monitoring of Wound Healing Using a Wearable Enzymatic Uric Acid Biosensor. J. Electrochem. Soc. 165, B3168 (2018).
Ibáñez-Redín, G. et al. Wearable potentiometric biosensor for analysis of urea in sweat. Biosens. Bioelectron. 223, 114994 (2023).
Xuan, X., Yoon, H. S. & Park, J. Y. A wearable electrochemical glucose sensor based on simple and low-cost fabrication supported micro-patterned reduced graphene oxide nanocomposite electrode on flexible substrate. Biosens. Bioelectron. 109, 75–82 (2018).
Chong, Y. W., Ismail, W., Ko, K. & Lee, C. Y. Energy Harvesting For Wearable Devices: A Review. IEEE Sens. J. 19, 9047–9062 (2019).
Mitcheson, P. D. in 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. 3432-3436. https://doi.org/10.1109/IEMBS.2010.5627952
Rasmussen, M., Abdellaoui, S. & Minteer, S. D. Enzymatic biofuel cells: 30 years of critical advancements. Biosens. Bioelectron. 76, 91–102 (2016).
Zhou, M. & Wang, J. Biofuel Cells for Self-Powered Electrochemical Biosensing and Logic Biosensing: A Review. Electroanalysis 24, 197–209 (2012).
Fu, L., Liu, J., Hu, Z. & Zhou, M. Recent Advances in the Construction of Biofuel Cells Based Self-powered Electrochemical Biosensors: A Review. Electroanalysis 30, 2535–2550 (2018).
He, R. et al. Flexible Miniaturized Sensor Technologies for Long-Term Physiological Monitoring. npj Flex. Electron. 6, 20 (2022).
Wang, J. et al. Ultra-Small Wearable Flexible Biosensor for Continuous Sweat Analysis. ACS Sens. 7, 3102–3107 (2022).
Cui, M., Chai, Z., Lu, Y., Zhu, J. & Chen, J. Developments of polyurethane in biomedical applications: A review. Resour. Chem. Mater. 2, 262–276 (2023).
He, Z. et al. Highly stretchable multi-walled carbon nanotube/thermoplastic polyurethane composite fibers for ultrasensitive, wearable strain sensors. Nanoscale 11, 5884–5890 (2019).
Slobodian, P., Danova, R., Olejnik, R., Matyas, J. & Münster, L. Multifunctional flexible and stretchable polyurethane/carbon nanotube strain sensor for human breath monitoring. Polym. Adv. Technol. 30, 1891–1898 (2019).
Choi, Y.-I. et al. Stretchable and transparent nanofiber-networked electrodes based on nanocomposites of polyurethane/reduced graphene oxide/silver nanoparticles with high dispersion and fused junctions. Nanoscale 11, 3916–3924 (2019).
Chung, M. et al. Fabrication of a Wearable Flexible Sweat pH Sensor Based on SERS-Active Au/TPU Electrospun Nanofibers. ACS Appl. Mater. Interfaces 13, 51504–51518 (2021).
Fang, Y. et al. Self-healable and recyclable polyurethane-polyaniline hydrogel toward flexible strain sensor. Compos. Part B: Eng. 219, 108965 (2021).
Bandodkar, A. J., Nuñez-Flores, R., Jia, W. & Wang, J. All-Printed Stretchable Electrochemical Devices. Adv. Mater. 27, 3060–3065 (2015).
Jeerapan, I., Sempionatto, J. R., Pavinatto, A., You, J.-M. & Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 4, 18342–18353 (2016). The paper demonstrates highly stretchable textile-based glucose and lactate biofuel cells, acting as self-powered sweat sensors, fabricated using screen-printing of customized stress-enduring inks.
Lee, H. et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016). The electrochemical device with thermoresponsive microneedles, featuring stretchable designs, provides conformal contacts to human skin under deformation for diabetes monitoring and therapy.
Bandodkar, A. J., Jeerapan, I., You, J.-M., Nuñez-Flores, R. & Wang, J. Highly Stretchable Fully-Printed CNT-Based Electrochemical Sensors and Biofuel Cells: Combining Intrinsic and Design-Induced Stretchability. Nano Lett. 16, 721–727 (2016). Highly stretchable printed carbon nanotube-based electrochemical sensors and biofuel cells were fabricated, capable of withstanding strains as high as 500% with negligible effects on their structural integrity and electrochemical performance.
Chen, X. et al. Stretchable and Flexible Buckypaper-Based Lactate Biofuel Cell for Wearable Electronics. Adv. Funct. Mater. 29, 1905785 (2019). The functionalized buckypaper electrodes are assembled onto a stretchable, screen-printed current collector with an “island-bridge” configuration, ensuring conformal contact between the wearable biofuel cell and the human body and endows the biofuel cell with good performance stability under stretching conditions.
Asaduzzaman, M. et al. A hybridized nano-porous carbon reinforced 3D graphene-based epidermal patch for precise sweat glucose and lactate analysis. Biosens. Bioelectron. 219, 114846 (2023). A functionalized hybridized nanoporous carbon-encapsulated flexible three-dimensional porous graphene enhanced the electrochemical surface area, heterogeneous electron transfer rate, and electrocatalytic activity for the detection of lactate, glucose, temperature, and pH in human perspiration.
Ania, C. O., Gomis-Berenguer, A., Dentzer, J. & Vix-Guterl, C. Nanoconfinement of glucose oxidase on mesoporous carbon electrodes with tunable pore sizes. J. Electroanalytical Chem. 808, 372–379 (2018).
Yoon, H. et al. A chemically modified laser-induced porous graphene based flexible and ultrasensitive electrochemical biosensor for sweat glucose detection. Sens. Actuators B: Chem. 311, 127866 (2020).
Xiao, X., Siepenkoetter, T., Conghaile, P. Ó., Leech, D. & Magner, E. Nanoporous Gold-Based Biofuel Cells on Contact Lenses. ACS Appl. Mater. Interfaces 10, 7107–7116 (2018). Mechanically stable and flexible nanoporous gold electrodes were prepared using an electrochemical dealloying method for the fabrication of lactate/O2 enzymatic biofuel cell.
Sun, M. et al. A flexible and wearable epidermal ethanol biofuel cell for on-body and real-time bioenergy harvesting from human sweat. Nano Energy 86, 106061 (2021). The enzymatic screen-printed electrode (SPE) arrays based on the three-dimensional coralloid nitrogen doped hierarchical-micromesoporous carbon aerogels (3D-NHCAs) was utilized for the fabrication of a flexible and wearable epidermal ethanol biofuel cell.
Qiu, H. et al. Enzyme-modified nanoporous gold-based electrochemical biosensors. Biosens. Bioelectron. 24, 3014–3018 (2009).
Aldea, A. et al. Gold coated electrospun polymeric fibres as new electrode platform for glucose oxidase immobilization. Microchemical J. 165, 106108 (2021).
Garland, N. T. et al. Wearable Flexible Perspiration Biosensors Using Laser-Induced Graphene and Polymeric Tape Microfluidics. ACS Appl. Mater. Interfaces 15, 38201–38213 (2023).
Li, M. et al. A highly integrated sensing paper for wearable electrochemical sweat analysis. Biosens. Bioelectron. 174, 112828 (2021).
Temoçin, Z. Designing of a stable and selective glucose biosensor by glucose oxidase immobilization on glassy carbon electrode sensitive to H2O2 via nanofiber interface. J. Appl. Electrochem. 51, 283–293 (2021).
Jirakunakorn, R. et al. Uric acid enzyme biosensor based on a screen-printed electrode coated with Prussian blue and modified with chitosan-graphene composite cryogel. Microchemical J. 154, 104624 (2020).
Lim, S., Jung, G. A., Glover, D. J. & Clark, D. S. Enhanced Enzyme Activity through Scaffolding on Customizable Self-Assembling Protein Filaments. Small 15, 1805558 (2019).
Kalyana Sundaram, S. D., Hossain, M. M., Rezki, M., Ariga, K. & Tsujimura, S. Enzyme Cascade Electrode Reactions with Nanomaterials and Their Applicability towards Biosensor and Biofuel Cells. Biosensors 13, 1018 (2023).
Macazo, F. C. & Minteer, S. D. Enzyme cascades in biofuel cells. Curr. Opin. Electrochem. 5, 114–120 (2017).
Guan, S. et al. A Dual-Functional MXene-Based Bioanode for Wearable Self-Charging Biosupercapacitors. Adv. Mater. 36, 2305854 (2024). MXene/single-walled carbon nanotube/lactate oxidase hierarchical structure is designed, which provides a superior three-dimensional catalytic microenvironment for enzyme accommodation to harvest energy from sweat.
Kwon, C. H. et al. High-power hybrid biofuel cells using layer-by-layer assembled glucose oxidase-coated metallic cotton fibers. Nat. Commun. 9, 4479 (2018). The paper demonstrates ultrahigh-power hybrid DET-BFCs that maximize the electrocatalytic activity on highly porous cotton fibers.
Reuillard, B. et al. High power enzymatic biofuel cell based on naphthoquinone-mediated oxidation of glucose by glucose oxidase in a carbon nanotube 3D matrix. Phys. Chem. Chem. Phys. 15, 4892–4896 (2013). A high-power enzymatic biofuel cell was demonstrated based on naphthoquinone-mediated oxidation of glucose by glucose oxidase in a carbon nanotube three-dimensional matrix.
Elouarzaki, K., Cheng, D., Fisher, A. C. & Lee, J.-M. Coupling orientation and mediation strategies for efficient electron transfer in hybrid biofuel cells. Nat. Energy 3, 574–581 (2018).
Ben Rejeb, K., Abdelly, C. & Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 80, 278–284 (2014).
van den Burg, B. Extremophiles as a source for novel enzymes. Curr. Opin. Microbiol. 6, 213–218 (2003).
Bollella, P. & Katz, E. Enzyme-Based Biosensors: Tackling Electron Transfer Issues. Sensors 20, https://doi.org/10.3390/s20123517 (2020).
Ittisoponpisan, S. & Jeerapan, I. In Silico Analysis of Glucose Oxidase from Aspergillus niger: Potential Cysteine Mutation Sites for Enhancing Protein Stability. Bioengineering 8, https://doi.org/10.3390/bioengineering8110188 (2021).
Lin, Y. et al. Porous Enzymatic Membrane for Nanotextured Glucose Sweat Sensors with High Stability toward Reliable Noninvasive Health Monitoring. Adv. Funct. Mater. 29, 1902521 (2019).
Jia, W.-Z., Wang, K. & Xia, X.-H. Elimination of electrochemical interferences in glucose biosensors. TrAC Trends Anal. Chem. 29, 306–318 (2010).
Mohammadzadeh Kakhki, R. Nafion based biosensors: a review of recent advances and applications. Int. J. Polymeric Mater. Polymeric Biomater. 1–18, https://doi.org/10.1080/00914037.2023.2297436.
Adachi, T., Kitazumi, Y., Shirai, O. & Kano, K. Development Perspective of Bioelectrocatalysis-Based Biosensors. Sensors 20, https://doi.org/10.3390/s20174826 (2020).
Jiang, Y. et al. Recent Advances of Prussian Blue-Based Wearable Biosensors for Healthcare. Anal. Chem. 94, 297–311 (2022).
Abdul-Aziz, A. & Wong, F.-L. Interference elimination of an amperometric glucose biosensor using poly(hydroxyethyl methacrylate) membrane. Eng. Life Sci. 11, 20–25 (2011).
Tchekep, A. G. K., Suryanarayanan, V. & K Pattanayak, D. Alternative approach for highly sensitive and free-interference electrochemical dopamine sensing. Carbon 204, 57–69 (2023).
Jayakumar, K. et al. Tethering zwitterionic polymer coatings to mediated glucose biosensor enzyme electrodes can decrease sensor foreign body response yet retain sensor sensitivity to glucose. Biosens. Bioelectron. 219, 114815 (2023).
Goda, T., Ishihara, K. & Miyahara, Y. Critical update on 2-methacryloyloxyethyl phosphorylcholine (MPC) polymer science. Journal of Applied Polymer Science 132, https://doi.org/10.1002/app.41766 (2015).
Sangsawang, R., Buranachai, C., Thavarungkul, P., Kanatharana, P. & Jeerapan, I. Cavitas electrochemical sensors for the direct determination of salivary thiocyanate levels. Microchimica Acta 188, 415 (2021).
Zhao, Z. et al. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring. Lab a Chip 21, 916–932 (2021).< /span>
Ma, B. et al. Wearable capillary microfluidics for continuous perspiration sensing. Talanta 212, 120786 (2020).
Mei, X., Yang, J., Liu, J. & Li, Y. Wearable, nanofiber-based microfluidic systems with integrated electrochemical and colorimetric sensing arrays for multiplex sweat analysis. Chem. Eng. J. 454, 140248 (2023).
Saha, T. et al. Wireless Wearable Electrochemical Sensing Platform with Zero-Power Osmotic Sweat Extraction for Continuous Lactate Monitoring. ACS Sens. 7, 2037–2048 (2022). A continuous sweat lactate monitoring platform was demonstrated by combining a hydrogel for osmotic sweat extraction, with a paper microfluidic channel for facilitating sweat transport and management.
Bae, C. W., Chinnamani, M. V., Lee, E. H. & Lee, N.-E. Stretchable Non-Enzymatic Fuel Cell-Based Sensor Patch Integrated with Thread-Embedded Microfluidics for Self-Powered Wearable Glucose Monitoring. Adv. Mater. Interfaces 9, 2200492 (2022). A stretchable non-enzymatic fuel cell-based sensor patch was demonstrated using cotton thread-embedded microfluidics for self-powered wearable glucose monitoring.
Vinoth, R., Nakagawa, T., Mathiyarasu, J. & Mohan, A. M. V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 6, 1174–1186 (2021).
Xiao, J. et al. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 91, 14803–14807 (2019).
Koh, A. et al. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 8, 366ra165 (2016).
Choi, J. et al. Soft, Skin-Integrated Multifunctional Microfluidic Systems for Accurate Colorimetric Analysis of Sweat Biomarkers and Temperature. ACS Sens. 4, 379–388 (2019).
Zhang, Y. et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab a Chip 19, 1545–1555 (2019).
Li, S., Ma, Z., Cao, Z., Pan, L. & Shi, Y. Advanced Wearable Microfluidic Sensors for Healthcare Monitoring. Small 16, 1903822 (2020).
Chen, G., Zheng, J., Liu, L. & Xu, L. Application of Microfluidics in Wearable Devices. Small Methods 3, 1900688 (2019).
Zhang, H. et al. Wearable microfluidic patch with integrated capillary valves and pumps for sweat management and multiple biomarker analysis. Biomicrofluidics 16, 044104 (2022).
Shajari, S. et al. MicroSweat: A Wearable Microfluidic Patch for Noninvasive and Reliable Sweat Collection Enables Human Stress Monitoring. Adv. Sci. 10, 2204171 (2023).
Yeo, J. C., Kenry & Lim, C. T. Emergence of microfluidic wearable technologies. Lab a Chip 16, 4082–4090 (2016).
Lin, H. et al. A programmable epidermal microfluidic valving system for wearable biofluid management and contextual biomarker analysis. Nat. Commun. 11, 4405 (2020).
Mishra, N. et al. A soft wearable microfluidic patch with finger-actuated pumps and valves for on-demand, longitudinal, and multianalyte sweat sensing. ACS Sens. 7, 3169–3180 (2022).
Yang, J. H., David, U., Noh, Y. S. & Koh, A. Dual-valved skin-interfaced microfluidic device for programmed time-control sweat collection. Sens. Actuators B: Chem. 395, 134441 (2023).
Scodeller, P. et al. Layer-by-Layer Self-Assembled Osmium Polymer-Mediated Laccase Oxygen Cathodes for Biofuel Cells: The Role of Hydrogen Peroxide. J. Am. Chem. Soc. 132, 11132–11140 (2010).
Brunel, L. et al. Oxygen transport through laccase biocathodes for a membrane-less glucose/O2 biofuel cell. Electrochem. Commun. 9, 331–336 (2007).
Cussler, E. L. Diffusion: mass transfer in fluid systems. (Cambridge university press, 2009).
Lei, Y., Sun, R., Zhang, X., Feng, X. & Jiang, L. Oxygen-Rich Enzyme Biosensor Based on Superhydrophobic Electrode. Adv. Mater. 28, 1477–1481 (2016).
Miyamoto, A. et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 12, 907–913 (2017).
Zhou, W. et al. Gas-Permeable, Ultrathin, Stretchable Epidermal Electronics with Porous Electrodes. ACS Nano 14, 5798–5805 (2020).
Liang, X. et al. Hydrophilic, Breathable, and Washable Graphene Decorated Textile Assisted by Silk Sericin for Integrated Multimodal Smart Wearables. Adv. Funct. Mater. 32, 2200162 (2022).
Liu, L. et al. Nanofiber-Reinforced Silver Nanowires Network as a Robust, Ultrathin, and Conformable Epidermal Electrode for Ambulatory Monitoring of Physiological Signals. Small 15, 1900755 (2019).
Qiu, Q. et al. Highly flexible, breathable, tailorable and washable power generation fabrics for wearable electronics. Nano Energy 58, 750–758 (2019).
Yan, X. et al. Highly breathable, surface-hydrophobic and wet-adhesive silk based epidermal electrode for long-term electrophysiological monitoring. Compos. Sci. Technol. 230, 109751 (2022).
Yang, S. et al. Permeable and washable electronics based on polyamide fibrous membrane for wearable applications. Compos. Sci. Technol. 207, 108729 (2021).
Brown, M. S. et al. Electronic-ECM: A Permeable Microporous Elastomer for an Advanced Bio-Integrated Continuous Sensing Platform. Adv. Mater. Technol. 5, 2000242 (2020).
Kang, Z. et al. A wearable and flexible lactic-acid/O2 biofuel cell with an enhanced air-breathing biocathode. Biosens. Bioelectron. 246, 115845 (2024). A superhydrophobic base electrode creating an efficient air-solid-liquid triphase interface is developed to improve the oxygen supply efficiency for the air-breathing biocathode.
Zhuo, J. et al. A breathable and woven hybrid energy harvester with optimized power management for sustainably powering electronics. Nano Energy 112, 108436 (2023). The paper describes a breathable and woven hybrid energy harvester with breathability, flexibility, and comfortability that was woven from a triboelectric nanogenerator and biofuel cells to sustainably power electronics.
Jeerapan, I., Sempionatto, J. R., You, J.-M. & Wang, J. Enzymatic glucose/oxygen biofuel cells: Use of oxygen-rich cathodes for operation under severe oxygen-deficit conditions. Biosens. Bioelectron. 122, 284–289 (2018). The oxygen-rich cathode was demonstrated using a polychlorotrifluoroethylene binder, which provides an internal oxygen supply for the BFC reduction reaction.
Wang, J., Chen, L. & Chatrathi, M.-P. Evaluation of different fluorocarbon oils for their internal oxygen supply in glucose microsensors operated under oxygen-deficit conditions. Analytica Chim. Acta 411, 187–192 (2000).
Rasitanon, N. et al. Redox-Mediated Gold Nanoparticles with Glucose Oxidase and Egg White Proteins for Printed Biosensors and Biofuel Cells. Int. J. Mol. Sci. 24, https://doi.org/10.3390/ijms24054657 (2023). The paper demonstrates the biointerface of using egg white proteins to prevent the escape of enzymes and provide a microenvironment for printed biosensors and biofuel cells.
Liu, X. et al. Coupling of Silk Fibroin Nanofibrils Enzymatic Membrane with Ultra-Thin PtNPs/Graphene Film to Acquire Long and Stable On-Skin Sweat Glucose and Lactate Sensing. Small Methods 5, 2000926 (2021). A bio-active porous enzymatic nanofiber membrane composed of silk fibroin nanofibrils and enzymes was developed to retain the enzymes for sweat glucose and lactate sensing.
Jeerapan, I. et al. Fully edible biofuel cells. J. Mater. Chem. B 6, 3571–3578 (2018).
Rafiq, K. et al. Fabrication of a highly effective electrochemical urea sensing platform based on urease-immobilized silk fibroin scaffold and aminated glassy carbon electrode. Sens. Actuators B: Chem. 251, 472–480 (2017).
Molinnus, D. et al. Towards a flexible electrochemical biosensor fabricated from biocompatible Bombyx mori silk. Biosens. Bioelectron. 183, 113204 (2021).
Lu, S. et al. Stabilization of Enzymes in Silk Films. Biomacromolecules 10, 1032–1042 (2009).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Wu, Z. et al. Interstitial fluid-based wearable biosensors for minimally invasive healthcare and biomedical applications. Commun. Mater. 5, 33 (2024).
Zhang, P. et al. Wearable transdermal colorimetric microneedle patch for Uric acid monitoring based on peroxidase-like polypyrrole nanoparticles. Analytica Chim. Acta 1212, 339911 (2022).
Kim, J. et al. Simultaneous Monitoring of Sweat and Interstitial Fluid Using a Single Wearable Biosensor Platform. Adv. Sci. 5, 1800880 (2018).
Goud, K. Y. et al. Wearable electrochemical microneedle sensing platform for real-time continuous interstitial fluid monitoring of apomorphine: Toward Parkinson management. Sens. Actuators B: Chem. 354, 131234 (2022).
Dai, Y. et al. Wearable Sensor Patch with Hydrogel Microneedles for In Situ Analysis of Interstitial Fluid. ACS Appl. Mater. Interfaces 15, 56760–56773 (2023).
Dervisevic, M. et al. Wearable microneedle array-based sensor for transdermal monitoring of pH levels in interstitial fluid. Biosens. Bioelectron. 222, 114955 (2023).
Li, J. et al. High-Performance Flexible Microneedle Array as a Low-Impedance Surface Biopotential Dry Electrode for Wearable Electrophysiological Recording and Polysomnography. Nano-Micro Lett. 14, 132 (2022).
Zhang, B. L., Yang, Y., Zhao, Z. Q. & Guo, X. D. A gold nanoparticles deposited polymer microneedle enzymatic biosensor for glucose sensing. Electrochim. Acta 358, 136917 (2020).
Kashaninejad, N. et al. Microneedle Arrays for Sampling and Sensing Skin Interstitial Fluid. Chemosensors 9, 83 (2021).
Saifullah, K. M. & Faraji Rad, Z. Sampling Dermal Interstitial Fluid Using Microneedles: A Review of Recent Developments in Sampling Methods and Microneedle-Based Biosensors. Adv. Mater. Interfaces 10, 2201763 (2023).
Parrilla, M. et al. Wearable Microneedle-Based Array Patches for Continuous Electrochemical Monitoring and Drug Delivery: Toward a Closed-Loop System for Methotrexate Treatment. ACS Sens. 8, 4161–4170 (2023).
Li, Z. et al. Electrochemical detection of cholesterol in human biofluid using microneedle sensor. J. Mater. Chem. B 11, 6075–6081 (2023).
Chinnamani, M. V. et al. Soft microfiber-based hollow microneedle array for stretchable microfluidic biosensing patch with negative pressure-driven sampling. Biosens. Bioelectron. 237, 115468 (2023).
Lee, D.-S., Li, C. G., Ihm, C. & Jung, H. A three-dimensional and bevel-angled ultrahigh aspect ratio microneedle for minimally invasive and painless blood sampling. Sens. Actuators B: Chem. 255, 384–390 (2018).
Goud, K. Y. et al. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 4, 2196–2204 (2019).
Li, H. et al. Microneedle-Based Potentiometric Sensing System for Continuous Monitoring of Multiple Electrolytes in Skin Interstitial Fluids. ACS Sens. 6, 2181–2190 (2021).
Mohan, A. M. V., Windmiller, J. R., Mishra, R. K. & Wang, J. Continuous minimally-invasive alcohol monitoring using microneedle sensor arrays. Biosens. Bioelectron. 91, 574–579 (2017).
Caliò, A. et al. Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens. Actuators B: Chem. 236, 343–349 (2016).
Bollella, P., Sharma, S., Cass, A. E. G. & Antiochia, R. Minimally-invasive Microneedle-based Biosensor Array for Simultaneous Lactate and Glucose Monitoring in Artificial Interstitial Fluid. Electroanalysis 31, 374–382 (2019).
De la Paz, E. et al. Non-invasive monitoring of interstitial fluid lactate through an epidermal iontophoretic device. Talanta 254, 124122 (2023).
Pikal, M. J. The role of electroosmotic flow in transdermal iontophoresis. Adv. Drug Deliv. Rev. 46, 281–305 (2001).
Gupta, D. K., Ahad, A., Aqil, M., Al-Mohizea, A. M. & Al-Jenoobi, F. I. Chapter 18 - Iontophoretic drug delivery: concepts, approaches, and applications in Advanced and Modern Approaches for Drug Delivery (eds Amit Kumar Nayak, Md Saquib Hasnain, Bibek Laha, & Sabyasachi Maiti) 515-546 (Academic Press, 2023). https://doi.org/10.1016/B978-0-323-91668-4.00016-2
Zheng, H. et al. Reverse iontophoresis with the development of flexible electronics: A review. Biosens. Bioelectron. 223, 115036 (2023).
Park, H., Park, W. & Lee, C. H. Electrochemically active materials and wearable biosensors for the in situ analysis of body fluids for human healthcare. NPG Asia Mater. 13, 23 (2021).
Chaudon, M. J., Hulea, O., Yakoub, A., Monnier, P. & Saadaoui, M. Wearable device for iontophoretic treatment and monitoring of pressure ulcers: Proof-of-concept. Med. Eng. Phys. 107, 103861 (2022).
Benoît, L., Richard, H. G. & Delgado-Charro, M. B. Reverse iontophoresis for non-invasive transdermal monitoring. Physiological Meas. 25, R35 (2004).
Cheng, Y. et al. A touch-actuated glucose sensor fully integrated with microneedle array and reverse iontophoresis for diabetes monitoring. Biosens. Bioelectron. 203, 114026 (2022).
Mohan, A. M. V., Rajendran, V., Mishra, R. K. & Jayaraman, M. Recent advances and perspectives in sweat based wearable electrochemical sensors. TrAC Trends Anal. Chem. 131, 116024 (2020).
Sempionatto, J. R. et al. Epidermal Enzymatic Biosensors for Sweat Vitamin C: Toward Personalized Nutrition. ACS Sens. 5, 1804–1813 (2020).
Mastropasqua, L. et al. Corneal Cross-linking: Intrastromal Riboflavin Concentration in Iontophoresis-Assisted Imbibition Versus Traditional and Transepithelial Techniques. Am. J. Ophthalmol. 157, 623–630.e621 (2014).
Fan, Q., Sirkar, K. K. & Michniak, B. Iontophoretic transdermal drug delivery system using a conducting polymeric membrane. J. Membr. Sci. 321, 240–249 (2008).
Hao, J., Smith, K. A. & Li, S. K. Chemical method to enhance transungual transport and iontophoresis efficiency. Int. J. Pharmaceutics 357, 61–69 (2008).
Kim, J. et al. Noninvasive Alcohol Monitoring Using a Wearable Tattoo-Based Iontophoretic-Biosensing System. ACS Sens. 1, 1011–1019 (2016).
Bandodkar, A. J. et al. Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat. Energy Environ. Sci. 10, 1581–1589 (2017).
Adekunle, A., Raghavan, V. & Tartakovsky, B. Real-time performance optimization and diagnostics during long-term operation of a solid anolyte microbial fuel cell biobattery. Batteries 5, 9 (2019).
Batchu, K., Probst, D., Satomura, T. & Sode, K. Development of the BioBattery: A novel enzyme fuel cell using a multicopper oxidase as an anodic enzyme. Biosens. Bioelectron. 252, 116092 (2024).
Yoshida, S. et al. Totally organic electrical skin patch powered by flexible biobattery. J. Phys.: Energy 2, 044004 (2020).
Zhang, J. et al. A wearable self-powered biosensor system integrated with diaper for detecting the urine glucose of diabetic patients. Sens. Actuators B: Chem. 341, 130046 (2021).
Yuan, Y. et al. Self-adhesive wearable poly (vinyl alcohol)-based hybrid biofuel cell powered by human bio-fluids. Biosens. Bioelectron. 247, 115930 (2024).
Lee, J., Han, S. & Kwon, Y. Self-charging hybrid energy devices collaborated with enzymatic biofuel cells and supercapacitors. Chem. Eng. J. 487, 150557 (2024).
Alam, F. et al. Lactate biosensing: The emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 117, 818–829 (2018).
Jo, S., Sung, D., Kim, S. & Koo, J. A review of wearable biosensors for sweat analysis. Biomed. Eng. Lett. 11, 117–129 (2021).
Komkova, M. A. et al. Simultaneous monitoring of sweat lactate content and sweat secretion rate by wearable remote biosensors. Biosens. Bioelectron. 202, 113970 (2022).
Alizadeh, A. et al. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab a Chip 18, 2632–2641 (2018).
Kumar, N., Lin, Y.-J., Huang, Y.-C., Liao, Y.-T. & Lin, S.-P. Detection of lactate in human sweat via surface-modified, screen-printed carbon electrodes. Talanta 265, 124888 (2023).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Abellán-Llobregat, A. et al. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 91, 885–891 (2017).
Cao, Q. et al. Three-dimensional paper-based microfluidic electrochemical integrated devices (3D-PMED) for wearable electrochemical glucose detection. RSC Adv. 9, 5674–5681 (2019).
Imani, S. et al. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 7, 11650 (2016).
Zahed, M. A. et al. A Nanoporous Carbon-MXene Heterostructured Nanocomposite-Based Epidermal Patch for Real-Time Biopotentials and Sweat Glucose Monitoring. Adv. Funct. Mater. 32, 2208344 (2022). The paper describes an epidermal patch for real-time biopotentials (ECG, EMG, EEG, and temperature) and sweat glucose monitoring using a nanoporous carbon-MXene heterostructured nanocomposite.
Xu, C. et al. A physicochemical-sensing electronic skin for stress response monitoring. Nat. Electronics, https://doi.org/10.1038/s41928-023-01116-6 (2024). An electronic skin that can monitor multiple stress-relevant biomarkers and can differentiate three stressors with the help of a machine learning pipeline was demonstrated for stress response assessment.
Zheng, X. T. et al. Carbon Dot-Doped Hydrogel Sensor Array for Multiplexed Colorimetric Detection of Wound Healing. ACS Appl. Mater. Interfaces 15, 17675–17687 (2023).
Tang, N. et al. Highly Efficient Self-Healing Multifunctional Dressing with Antibacterial Activity for Sutureless Wound Closure and Infected Wound Monitoring. Adv. Mater. 34, 2106842 (2022).
Garland, N. T. et al. A Miniaturized, Battery-Free, Wireless Wound Monitor That Predicts Wound Closure Rate Early. Adv. Healthc. Mater. 12, 2301280 (2023).
Sim, P., Strudwick, X. L., Song, Y., Cowin, A. J. & Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int J. Mol. Sci. 23, 13655 (2022).
Tang, N. et al. Wearable sensors and systems for wound healing-related pH and temperature detection. Micromachines 12, 243 (2021).
Sharp, D. & Davis, J. Integrated urate sensors for detecting wound infection. Electrochem. Commun. 10, 709–713 (2008).
Sharp, D. Printed composite electrodes for in-situ wound pH monitoring. Biosens. Bioelectron. 50, 399–405 (2013).
Shirzaei Sani, E. et al. A stretchable wireless wearable bioelectronic system for multiplexed monitoring and combination treatment of infected chronic wounds. Sci. Adv. 9, eadf7388 (2023).
Xu, G. et al. Battery-Free and Wireless Smart Wound Dressing for Wound Infection Monitoring and Electrically Controlled On-Demand Drug Delivery. Adv. Funct. Mater. 31, 2100852 (2021). The paper shows battery-free and wireless smart wound dressing for wound infection monitoring and electrically controlled on-demand drug delivery.
Meng, L., Liu, S., Borsa, B. A., Eriksson, M. & Mak, W. C. A conducting polymer-based array with multiplex sensing and drug delivery capabilities for smart bandages. Commun. Mater. 5, 28 (2024). The paper presents a smart theranostic bandage-based on conducting polymers for multiplex sensing and drug delivery capabilities.
Jourdan, T., Debs, N. & Frindel, C. The contribution of machine learning in the validation of commercial wearable sensors for gait monitoring in patients: a systematic review. Sensors 21, 4808 (2021).
Pires, I. M., Garcia, N. M., Pombo, N., Flórez-Revuelta, F. & Rodríguez, N. D. Validation Techniques for Sensor Data in Mobile Health Applications. J. Sens. 2016, 2839372 (2016).
Liu, Z.-P. Identifying network-based biomarkers of complex diseases from high-throughput data. Biomark. Med. 10, 633–650 (2016).
Zhang, J. et al. Recent advances in acoustic wave biosensors for the detection of disease-related biomarkers: A review. Analytica Chim. Acta 1164, 338321 (2021).
Hou, J., Liu, X., Hou, C., Huo, D. & Li, J. A PVDF-based colorimetric sensor array for noninvasive detection of multiple disease-related volatile organic compounds. Anal. Bioanal. Chem. 415, 6647–6661 (2023).
Dai, N. et al. Recent advances in wearable electromechanical sensors—Moving towards machine learning-assisted wearable sensing systems. Nano Energy 105, 108041 (2023).
Yazdanpanah, S., Shojae Chaeikar, S. & Jolfaei, A. Monitoring the security of audio biomedical signals communications in wearable IoT healthcare. Digital Commun. Netw. 9, 393–399 (2023).
Wang, C., He, T., Zhou, H., Zhang, Z. & Lee, C. Artificial intelligence enhanced sensors - enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 9, 17 (2023).
Chen, M., Cui, D., Haick, H. & Tang, N. Artificial Intelligence-Based Medical Sensors for Healthcare System. Adv. Sens. Res. 3, 2300009 (2024).
Rasheed, S. et al. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends Anal. Chem. 173, 117640 (2024).
Kant, T. et al. Progress in the design of portable colorimetric chemical sensing devices. Nanoscale 15, 19016–19038 (2023).
Wen, F. et al. Advances in chemical sensing technology for enabling the next-generation self-sustainable integrated wearable system in the IoT era. Nano Energy 78, 105155 (2020).
Olzhabay, Y., Ng, A. & Ukaegbu, I. A. Perovskite PV energy harvesting system for uninterrupted IoT device applications. Energies 14, 7946 (2021).
Gao, Y., Cho, J. H., Ryu, J. & Choi, S. A scalable yarn-based biobattery for biochemical energy harvesting in smart textiles. Nano Energy 74, 104897 (2020).
Ryu, J., Gao, Y., Cho, J. H. & Choi, S. Horizontally structured microbial fuel cells in yarns and woven fabrics for wearable bioenergy harvesting. J. Power Sources 484, 229271 (2021).
Kim, J., Campbell, A. S., de Ávila, B. E.-F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Tu, J., Torrente-Rodríguez, R. M., Wang, M. & Gao, W. The Era of Digital Health: A Review of Portable and Wearable Affinity Biosensors. Adv. Funct. Mater. 30, 1906713 (2020).
Yang, B., Li, J., Deng, H. & Zhang, L. Progress of Mimetic Enzymes and Their Applications in Chemical Sensors. Crit. Rev. Anal. Chem. 46, 469–481 (2016).
Nasir, M. et al. An overview on enzyme-mimicking nanomaterials for use in electrochemical and optical assays. Microchimica Acta. 184, 323–342 (2017).
Calero, D., Paul, S., Gesing, A., Alves, F. & Cordioli, J. A. A technical review and evaluation of implantable sensors for hearing devices. Biomed. Eng. OnLine 17, 23 (2018).
Ashammakhi, N. et al. Biodegradable Implantable Sensors: Materials Design, Fabrication, and Applications. Adv. Funct. Mater. 31, 2104149 (2021).
Min, J., Sempionatto, J. R., Teymourian, H., Wang, J. & Gao, W. Wearable electrochemical biosensors in North America. Biosens. Bioelectron. 172, 112750 (2021).
Yang, B., Jiang, X., Fang, X. & Kong, J. Wearable chem-biosensing devices: from basic research to commercial market. Lab a Chip 21, 4285–4310 (2021).
Xia, Y. et al. Wearable electrochemical sensor based on bimetallic MOF coated CNT/PDMS film electrode via a dual-stamping method for real-time sweat glucose analysis. Analytica Chim. Acta. 1278, 341754 (2023).
Han, J. et al. Pt-poly(L-lactic acid) microelectrode-based microsensor for in situ glucose detection in sweat. Biosens. Bioelectron. 170, 112675 (2020).
Wang, Y. et al. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 198, 86–92 (2019).
Chen, Q. et al. Silk-Based Electrochemical Sensor for the Detection of Glucose in Sweat. Biomacromolecules 23, 3928–3935 (2022).
Hozumi, S., Honda, S., Arie, T., Akita, S. & Takei, K. Multimodal Wearable Sensor Sheet for Health-Related Chemical and Physical Monitoring. ACS Sens. 6, 1918–1924 (2021).
Shu, Y. et al. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 93, 16222–16230 (2021).
Oh, S. Y. et al. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 10, 13729–13740 (2018).
Liu, Y., Zhong, L., Zhang, S., Wang, J. & Liu, Z. An ultrasensitive and wearable photoelectrochemical sensor for unbiased and accurate monitoring of sweat glucose. Sens. Actuators B: Chem. 354, 131204 (2022).
Toi, P. T., Trung, T. Q., Dang, T. M. L., Bae, C. W. & Lee, N.-E. Highly Electrocatalytic, Durable, and Stretchable Nanohybrid Fiber for On-Body Sweat Glucose Detection. ACS Appl. Mater. Interfaces 11, 10707–10717 (2019).
Xuan, X. et al. Fully Integrated Wearable Device for Continuous Sweat Lactate Monitoring in Sports. ACS Sens. 8, 2401–2409 (2023).
Wu, Z.-Q., Cao, X.-Q., Hua, Y. & Yu, C.-M. A Bifunctional Wearable Sensor Based on a Nanoporous Membrane for Simultaneous Detection of Sweat Lactate and Temperature. Analytical Chem. https://doi.org/10.1021/acs.analchem.3c05216 (2024).
Zhao, Z. et al. Core-shell structured gold nanorods on thread-embroidered fabric-based microfluidic device for Ex Situ detection of glucose and lactate in sweat. Sens. Actuators B: Chem. 353, 131154 (2022).
Zhu, C. et al. A flexible electrochemical biosensor based on functionalized poly(3,4-ethylenedioxythiophene) film to detect lactate in sweat of the human body. J. Colloid Interface Sci. 617, 454–462 (2022).
Wang, R., Zhai, Q., An, T., Gong, S. & Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 222, 121484 (2021).
Xuan, X., Pérez-Ràfols, C., Chen, C., Cuartero, M. & Crespo, G. A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 6, 2763–2771 (2021).
Jiang, D. et al. In-situ preparation of lactate-sensing membrane for the noninvasive and wearable analysis of sweat. Biosens. Bioelectron. 210, 114303 (2022).
Huang, X. et al. Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Des. Manuf. 5, 201–209 (2022).
Luo, X. et al. Wearable Tape-Based Smart Biosensing Systems for Lactate and Glucose. IEEE Sens. J. 20, 3757–3765 (2020).
Luo, X. et al. Wearable Carbon Nanotube-Based Biosensors on Gloves for Lactate. Sensors 18, https://doi.org/10.3390/s18103398 (2018).
Bae, C. W. et al. Fully Stretchable Capillary Microfluidics-Integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Appl. Mater. Interfaces 11, 14567–14575 (2019).
Acknowledgements
We would like to thank the Talent Management Project of Prince of Songkla University. We also gratefully acknowledge the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research, and Innovation (MHESI).
Author information
Authors and Affiliations
Contributions
I.J. and S.K. conceptualized and wrote the article and created figures.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Peer review
Peer review information
Communications Materials thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Rona Chandrawati & John Plummer.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khumngern, S., Jeerapan, I. Synergistic convergence of materials and enzymes for biosensing and self-sustaining energy devices towards on-body health monitoring. Commun Mater 5, 135 (2024). https://doi.org/10.1038/s43246-024-00557-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00557-6
- Springer Nature Limited