Abstract
A fundamentally novel function proposed for extracellular vesicles (EVs) is to transfer bioactive molecules in intercellular signaling. In this minireview, we discuss recent progress on EV-mediated cargo transfer in the central nervous system (CNS) and major gaps in previous studies. We also suggest a set of experiments necessary for bridging the gaps and establishing the physiological roles of EV-mediated cargo transfer.
Similar content being viewed by others
Introduction
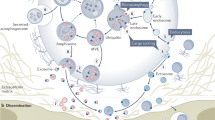
EVs are membranous particles released by virtually all cell types including neurons and glial cells of the CNS1,2,3,4. EVs can cross the blood-brain barrier under certain conditions such that CNS-originating EVs can enter the circulation and reach other tissues5,6,7. Likewise, CNS-resident EVs may come from tissues outside the CNS. EVs enable neurons and glial cells to eliminate excess or harmful membranes and macromolecules8,9,10,11. EVs also mediate intercellular signaling when EV-anchored ligands interact with their receptors displayed on the surfaces of recipient cells12,13,14,15. However, the most fascinating function proposed for EVs is the mediation of intercellular signaling via the transfer of bioactive molecules including proteins, RNAs, lipids, and even entire organelles (Fig. 1 and Box 1)8,16,17,18.
EV-mediated intercellular cargo transfer is comprised of three sequential steps: EV release from donor cells, EV uptake into recipient cells, and delivery of EV-carried cargoes into the cytosol of recipient cells (Fig. 1)1. EV release and entry can be both stimulated by neuronal activities7,19,20,21. In the CNS, cargo transfer can occur between the same or different cell types4,22,23,24,25,26,27. The molecular basis of EV release from donor cells is relatively well understood: exosomes are formed by the exocytosis of intraluminal vesicles encapsulated in multivesicular bodies whereas microvesicles are released through direct budding from the plasma membrane1,28,29. Delivery of EV cargoes into recipient cells, however, remains poorly understood.
After uptake, the default route of EVs within recipient cells is lysosomal degradation (Fig. 1)1,2,30. To avoid degradation, EVs must fuse with the endosomal membrane following internalization or fuse directly with the plasma membrane of recipient cells (Fig. 1). After fusion, EV-carried lumenal cargoes are released to the cytosol whereas membrane proteins and lipids are integrated into the endomembranes of recipient cells25,31,32,33,34. Notably, EVs enable RNAs to serve as intercellular signaling messengers by shielding them from extracellular RNases. EV-delivered mRNAs are translated into signaling molecules25,26, whereas miRNAs modulate the expression of target genes in recipient cells35,36. EV-mediated transfer of entire mitochondria has been suggested to alter the metabolic states of recipient cells in the CNS37,38,39. Finally, the EV-mediated transfer pathway can also serve to spread disease-promoting molecules including tau, amyloid β peptide, and prion proteins40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55.
Here, we review evidence for EV-mediated cargo delivery in the CNS. We discuss major gaps in previous studies and outline experiments to examine the physiological roles of EV-mediated cargo transfer. EV release and EV signaling through ligand-receptor interactions (i.e., without cytosolic cargo delivery) are not covered here because they are well established and have been extensively discussed elsewhere1,4,5,8,9.
EV-mediated cargo transfer in intercellular signaling—progress and gaps in previous studies
In the past two decades, EV-mediated cargo transfer has been reported in many aspects of CNS physiology4,8,22. A typical study of EV-mediated cargo transfer begins with a signaling event elicited by incubation with purified EVs, including changes in cell growth, morphology, metabolism, or gene expression (e.g., mRNA translation). The cellular response is diminished when EV release from donor cells is reduced or when a putative cargo is deleted26,27,56. A notable example is oligodendrocyte-derived EVs that deliver bioactive proteins into neurons to induce metabolic changes56,57,58. These studies present exciting observations with the potential to radically change our view of CNS functions. However, despite the progress, much is still unknown about EV-mediated cargo delivery in the CNS.
First and foremost, studies of CNS EVs rarely examined cargo delivery to the cytosol of recipient cells. For most signaling functions proposed for EVs, substantial amounts of cargo need to be delivered into the cytosol of recipient cells. In particular, miRNA levels are extremely low in EVs and mammalian cells lack a miRNA-amplifying mechanism59,60. Hence, a large number of EV fusion events are required to deliver sufficient miRNA molecules to trigger cellular responses in recipient cells. Previous research, however, usually measured crude EV internalization without distinguishing cargoes delivered to the cytosol from those still trapped in the endosome/lysosome. Limited experiments carried out using cultured neuronal and non-neuronal cells showed little or no cytosolic delivery of EV cargoes61,62,63,64,65. Likewise, cytosolic cargo delivery was detected at very low levels in the CNS using animal models24. The low cytosolic delivery efficiency could be due to a mismatch of EVs and recipient cells (i.e., a non-physiological pair) and efficient cargo delivery could occur when EVs are matched with their cognate cell types. Such physiological cognate pairs remain to be definitively established for the CNS.
Previous studies often sought to determine the EV cargo(es) responsible for eliciting a signaling event, but the evidence was often insufficient. Deletion of a putative EV cargo such as a miRNA from donor cells does not provide a definitive answer because other EV cargoes could be altered as well. Thus, it is difficult to rule out the possibility that a signaling event is mediated by EV ligand-receptor interactions instead of cytosolic cargo delivery. Moreover, a cellular response could be triggered by a soluble molecule co-purified but not associated with EVs. Membrane-free particles often co-purify with EVs and it is challenging to fully separate them66. This issue was often addressed by reducing EV release from donor cells using pharmacological or genetic approaches51,67,68. When a signaling event was blunted, the data were often taken as evidence for a role of EVs in the pathway. However, reduction of EV release invariably compromises many other cellular pathways including secretion of non-EV molecules, thus precluding accurate assessment of EV’s role in an intercellular signaling event.
Moving forward—experiments to examine the physiological roles of EV-mediated cargo transfer
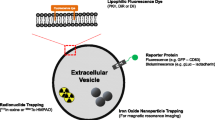
To fill the gaps in the studies of EV-mediated cargo transfer, it is crucial to directly examine cargo delivery into the cytosol of recipient cells. Highly sensitive assays have already been developed to detect cytosolic cargo delivery using genetically encoded reporters24,63,69,70. For instance, Cre proteins or mRNAs can be readily loaded into EVs when a Cre-encoding gene is expressed in donor cells. When delivered to the cytosol of recipient cells, Cre induces recombination of a floxed reporter gene and activates the expression of the reporter24,27,70. A major advantage of the Cre/LoxP system is its permanent recording of cytosolic delivery events. Other delivery assays take advantage of engineered luciferase or fluorescent proteins genetically loaded into EVs in donor cells63,71, permitting quantitative measurements of cytosolic delivery events. If EVs mediate an intercellular signaling event through transferring cargoes, it can be inferred that cytosolic cargo delivery occurs efficiently. Retroviruses are often used to stably express EV cargoes in reporter assays61,62. To preclude the possibility that EV-mediated cargo delivery is driven by residual viral fusion proteins, neutralizing antibodies against viral fusion proteins should be used as a control. Similarly, if donor cells are transfected with DNA plasmids encoding EV cargoes, caution needs to be taken to remove residual DNA and transfection reagents from purified EVs.
If efficient cytosolic cargo delivery is observed, a study needs to determine the cargo(es) responsible for a cellular response. This is a daunting task because as stated above, deletion of a cargo such as a miRNA in donor cells may alter other EV cargoes. This concern could be partially addressed by measuring the protein and RNA profiles of the mutant EVs using proteomics and RNA sequencing. Based on the outcomes of protein and RNA profiling, additional EV cargoes may need to be tested. Moreover, it is critical to adopt stringent EV isolation procedures including density gradient separation and size-exclusion chromatography. To further examine the roles of EVs in a signaling event, EVs or EV subpopulations could be immunodepleted from a sample using antibodies against EV surface markers. In parallel, EV membranes could be disrupted using sonication or detergents (followed by detergent removal).
The above experiments are essential to interrogate the physiological roles of EV-mediated cargo transfer, but the data obtained are correlative in nature. Ultimately, to definitively address the question, the fusogen mediating EV-cell fusion needs to be identified and perturbed at the molecular level. Like the entry of many enveloped viruses, EV-mediated cargo delivery at the endosome is pH dependent and sensitive to negative regulators of viral fusion63,71. Thus, it is tempting to postulate that the fusion of EVs with recipient cells is driven by a fusogen functionally resembling viral fusion proteins. An EV fusogen might be anchored only on EVs, similar to viral fusion proteins, while recipient cells only provide a receptor without directly contributing to the force-generating fusion machinery. Alternatively, an EV fusogen could be a trans-complex formed by proteins rooted in both the EV and recipient cell, analogous to the functions of HAP2 in cell-cell fusion and SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors) in intracellular vesicle fusion72,73,74. There could be multiple isoforms of an EV fusogen with distinct tissue distributions, enabling EVs to specifically deliver cargoes into their cognate recipient cells. The machinery mediating EV fusion with the plasma membrane may be the same or different from the machinery underlying EV-endosome fusion.
Once the EV fusogen is known, its activity can be selectively disrupted using gene knockout and dominant-negative mutants to determine the role of EV-mediated cargo delivery in an intercellular signaling event. If EV fusogens remain elusive after extensive efforts, an alternative possibility needs to be considered: EVs fuse with recipient cells through spontaneous lipid rearrangements without involving proteinaceous fusogens. Testing this model would require significant conceptual and technical innovations because all known biological membrane fusion processes are driven by proteinaceous fusogens.
Conclusion and perspectives
Despite the controversies and uncertainties associated with EV biology, the potential impacts of the field on fundamental biology and therapeutic delivery are immense. If validated, the significance of EV-mediated cargo transfer would be as high as that of intracellular membrane trafficking, which has been recognized by multiple Nobel Prizes including the 2013 Nobel Prize in Physiology or Medicine. The experiments outlined in this minireview are challenging but critical for establishing the physiological roles of EV-mediated cargo transfer in intercellular signaling. While this minireview focuses on the CNS, all the concepts are applicable to other cell types as well.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
References
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Thery, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
O’Brien, K., Breyne, K., Ughetto, S., Laurent, L. C. & Breakefield, X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Maas, S. L. N., Breakefield, X. O. & Weaver, A. M. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 27, 172–188 (2017).
Holm, M. M., Kaiser, J. & Schwab, M. E. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 41, 360–372 (2018).
Lizarraga-Valderrama, L. R. & Sheridan, G. K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 595, 1391–1410 (2021).
Kawahara, H. & Hanayama, R. The role of exosomes/extracellular vesicles in neural signal transduction. Biol. Pharm. Bull. 41, 1119–1125 (2018).
Chivet, M. et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J. Extracell. Vesicles 3, 24722 (2014).
Budnik, V., Ruiz-Canada, C. & Wendler, F. Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 17, 160–172 (2016).
Schnatz, A., Muller, C., Brahmer, A. & Kramer-Albers, E. M. Extracellular Vesicles in neural cell interaction and CNS homeostasis. FASEB Bioadv 3, 577–592 (2021).
Davis, C. H. et al. Transcellular degradation of axonal mitochondria. Proc. Natl Acad. Sci. USA 111, 9633–9638 (2014).
Takahashi, A. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 8, 15287 (2017).
Tkach, M. et al. Extracellular vesicles from triple negative breast cancer promote pro-inflammatory macrophages associated with better clinical outcome. Proc. Natl Acad. Sci. USA 119, e2107394119 (2022).
Gong, J., Korner, R., Gaitanos, L. & Klein, R. Exosomes mediate cell contact-independent ephrin-Eph signaling during axon guidance. J. Cell Biol. 214, 35–44 (2016).
Cossetti, C. et al. Extracellular vesicles from neural stem cells transfer IFN-gamma via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell 56, 193–204 (2014).
Ricklefs, F. L. et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 4, eaar2766 (2018).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Korkut, C. et al. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron 77, 1039–1046 (2013).
Antonucci, F. et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 31, 1231–1240 (2012).
Kur, I. M. et al. Neuronal activity triggers uptake of hematopoietic extracellular vesicles in vivo. PLoS Biol. 18, e3000643 (2020).
Faure, J. et al. Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 31, 642–648 (2006).
Goldie, B. J. et al. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 42, 9195–9208 (2014).
Gassama, Y. & Favereaux, A. Emerging roles of extracellular vesicles in the central nervous system: physiology, pathology, and therapeutic perspectives. Front Cell Neurosci. 15, 626043 (2021).
Fitzner, D. et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 124, 447–458 (2011).
Ridder, K. et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 12, e1001874 (2014).
Ashley, J. et al. Retrovirus-like gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell 172, 262–274.e211 (2018).
Pastuzyn, E. D. et al. The neuronal gene arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell 172, 275–288.e218 (2018).
Fruhbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 (2013).
van Niel, G., D’Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
van Niel, G. et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382 (2022).
Kanada, M. et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc. Natl Acad. Sci. USA 112, E1433–1442 (2015).
Vilcaes, A. A., Chanaday, N. L. & Kavalali, E. T. Interneuronal exchange and functional integration of synaptobrevin via extracellular vesicles. Neuron 109, 971–983.e975 (2021).
Grapp, M. et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat. Commun. 4, 2123 (2013).
Hervera, A. et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 20, 307–319 (2018).
Korkut, C. et al. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell 139, 393–404 (2009).
Zhang, Y. et al. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548, 52–57 (2017).
Simeoli, R. et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 8, 1778 (2017).
Park, J. H. & Hayakawa, K. Extracellular mitochondria signals in CNS disorders. Front Cell Dev. Biol. 9, 642853 (2021).
Hayakawa, K. et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555 (2016).
Peruzzotti-Jametti, L. et al. Neural stem cells traffic functional mitochondria via extracellular vesicles. PLoS Biol. 19, e3001166 (2021).
Goetzl, E. J. et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 30, 4141–4148 (2016).
Goetzl, E. J. et al. Cargo proteins of plasma astrocyte-derived exosomes in Alzheimer’s disease. FASEB J. 30, 3853–3859 (2016).
Coleman, B. M. & Hill, A. F. Extracellular vesicles—their role in the packaging and spread of misfolded proteins associated with neurodegenerative diseases. Semin Cell Dev. Biol. 40, 89–96 (2015).
Polanco, J. C., Li, C., Durisic, N., Sullivan, R. & Gotz, J. Exosomes taken up by neurons hijack the endosomal pathway to spread to interconnected neurons. Acta Neuropathol. Commun. 6, 10 (2018).
Baker, S., Polanco, J. C. & Gotz, J. Extracellular vesicles containing P301L mutant tau accelerate pathological tau phosphorylation and oligomer formation but do not seed mature neurofibrillary tangles in ALZ17 mice. J. Alzheimers Dis. 54, 1207–1217 (2016).
Saman, S. et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J. Biol. Chem. 287, 3842–3849 (2012).
Polanco, J. C., Scicluna, B. J., Hill, A. F. & Gotz, J. Extracellular vesicles isolated from the brains of rTg4510 mice seed tau protein aggregation in a threshold-dependent manner. J. Biol. Chem. 291, 12445–12466 (2016).
Rajendran, L. et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc. Natl Acad. Sci. USA 103, 11172–11177 (2006).
Eitan, E. et al. Extracellular vesicle-associated abeta mediates trans-neuronal bioenergetic and Ca(2+)-handling deficits in Alzheimer’s disease models. NPJ Aging Mech Dis. 2 https://doi.org/10.1038/npjamd.2016.19 (2016).
Jan, A. T. et al. Perspective insights of exosomes in neurodegenerative diseases: a critical appraisal. Front Aging Neurosci. 9, 317 (2017).
Dinkins, M. B., Dasgupta, S., Wang, G., Zhu, G. & Bieberich, E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 35, 1792–1800 (2014).
Asai, H. et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 18, 1584–1593 (2015).
Fevrier, B. et al. Cells release prions in association with exosomes. Proc. Natl Acad. Sci. USA 101, 9683–9688 (2004).
Vella, L. J. et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J. Pathol. 211, 582–590 (2007).
Emmanouilidou, E. et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J. Neurosci. 30, 6838–6851 (2010).
Feiler, M. S. et al. TDP-43 is intercellularly transmitted across axon terminals. J. Cell Biol. 211, 897–911 (2015).
Chamberlain, K. A. et al. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 109, 3456–3472.e3458 (2021).
Kramer-Albers, E. M. Superfood for axons: Glial exosomes boost axonal energetics by delivery of SIRT2. Neuron 109, 3397–3400 (2021).
Mukherjee, C. et al. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab. 32, 259–272.e210 (2020).
Albanese, M. et al. MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genet. 17, e1009951 (2021).
Gruner, H. N. & McManus, M. T. Examining the evidence for extracellular RNA function in mammals. Nat. Rev. Genet. 22, 448–458 (2021).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021).
Zhang, X. et al. Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev. Cell 55, 784–801.e789 (2020).
Bonsergent, E. et al. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat. Commun. 12, 1864 (2021).
de Jong, O. G. et al. A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat. Commun. 11, 1113 (2020).
Verweij, F. J. et al. Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev. Cell 48, 573–589.e574 (2019).
Auber, M., Frohlich, D., Drechsel, O., Karaulanov, E. & Kramer-Albers, E. M. Serum-free media supplements carry miRNAs that co-purify with extracellular vesicles. J. Extracell. Vesicles 8, 1656042 (2019).
Ostrowski, M. et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 (2010). sup 11-13.
Hsu, C. et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232 (2010).
O’Brien, K., Ughetto, S., Mahjoum, S., Nair, A. V. & Breakefield, X. O. Uptake, functionality, and re-release of extracellular vesicle-encapsulated cargo. Cell Rep. 39, 110651 (2022).
Zomer, A. et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015).
Joshi, B. S., de Beer, M. A., Giepmans, B. N. G. & Zuhorn, I. S. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 14, 4444–4455 (2020).
Sudhof, T. C. & Rothman, J. E. Membrane fusion: grappling with SNARE and SM proteins. Science 323, 474–477 (2009).
Fedry, J. et al. The ancient gamete fusogen HAP2 is a eukaryotic class II fusion protein. Cell 168, 904–915.e910 (2017).
Valansi, C. et al. Arabidopsis HAP2/GCS1 is a gamete fusion protein homologous to somatic and viral fusogens. J. Cell Biol. 216, 571–581 (2017).
Kramer-Albers, E. M. Extracellular vesicles at CNS barriers: mode of action. Curr. Opin. Neurobiol. 75, 102569 (2022).
Saint-Pol, J., Gosselet, F., Duban-Deweer, S., Pottiez, G. & Karamanos, Y. Targeting and Crossing the Blood-Brain Barrier with Extracellular Vesicles. Cells 9. https://doi.org/10.3390/cells9040851 (2020).
Acknowledgements
The authors thank Drs. Gregory Lavieu, Xuedong Liu, and Haijia Yu for insightful comments. This work was supported by National Institutes of Health (NIH) grants AG061829 (JS and MS), GM126960 (JS), and DK124431 (JS), and a University of Colorado ABNexus seed grant.
Author information
Authors and Affiliations
Contributions
J.S. conceptualized the review with inputs from C.W. and M.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Eva-Maria Krämer-Albers and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Eve Rogers.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, C., Stowell, M.H.B. & Shen, J. Progress and gaps of extracellular vesicle-mediated intercellular cargo transfer in the central nervous system. Commun Biol 5, 1223 (2022). https://doi.org/10.1038/s42003-022-04050-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-022-04050-z
- Springer Nature Limited
This article is cited by
-
Mechanisms of axonal support by oligodendrocyte-derived extracellular vesicles
Nature Reviews Neuroscience (2023)