Abstract
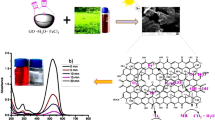
In the current arena, new-generation functional nanomaterials are the key players for smart solutions and applications including environmental decontamination of pollutants. Among the plethora of new-generation nanomaterials, graphene-based nanomaterials and nanocomposites are in the driving seat surpassing their counterparts due to their unique physicochemical characteristics and superior surface chemistry. The purpose of the present research was to synthesize and characterize magnetite iron oxide/reduced graphene oxide nanocomposites (FeNPs/rGO) via a green approach and test its application in the degradation of methylene blue. The modified Hummer's protocol was adopted to synthesize graphene oxide (GO) through a chemical exfoliation approach using a graphitic route. Leaf extract of Azadirachta indica was used as a green reducing agent to reduce GO into reduced graphene oxide (rGO). Then, using the green deposition approach and Azadirachta indica leaf extract, a nanocomposite comprising magnetite iron oxides and reduced graphene oxide i.e., FeNPs/rGO was synthesized. During the synthesis of functionalized FeNPs/rGO, Azadirachta indica leaf extract acted as a reducing, capping, and stabilizing agent. The final synthesized materials were characterized and analyzed using an array of techniques such as scanning electron microscopy (SEM)-energy dispersive X-ray microanalysis (EDX), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction analysis, and UV–visible spectrophotometry. The UV–visible spectrum was used to evaluate the optical characteristics and band gap. Using the FT-IR spectrum, functional groupings were identified in the synthesized graphene-based nanomaterials and nanocomposites. The morphology and elemental analysis of nanomaterials and nanocomposites synthesized via the green deposition process were investigated using SEM–EDX. The GO, rGO, FeNPs, and FeNPs/rGO showed maximum absorption at 232, 265, 395, and 405 nm, respectively. FTIR spectrum showed different functional groups (OH, COOH, C=O), C–O–C) modifying material surfaces. Based on Debye Sherrer's equation, the mean calculated particle size of all synthesized materials was < 100 nm (GO = 60–80, rGO = 90–95, FeNPs = 70–90, Fe/GO = 40–60, and Fe/rGO = 80–85 nm). Graphene-based nanomaterials displayed rough surfaces with clustered and spherical shapes and EDX analysis confirmed the presence of both iron and oxygen in all the nanocomposites. The final nanocomposites produced via the synthetic process degraded approximately 74% of methylene blue. Based on the results, it is plausible to conclude that synthesized FeNPs/rGO nanocomposites can also be used as a potential photocatalyst degrader for other different dye pollutants due to their lower band gap.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Global freshwater resources are limited and declining day by day. Water quality is challenged due to point and non-point sources of pollution. Freshwater resources are contaminated with industrial discharges/effluents and domestic wastes with nuisance organic and inorganic pollutants such as polyaromatic hydrocarbons (PAHs), persistent organic compounds (POPs), dyes, metals, and metalloids1,2. For safe and clean water, noxious pollutants should be removed from water by economically viable and efficient approaches. Rapid population expansion, increasing industrialization, urbanization, and pervasive agricultural practices have produced wastewater, which has rendered the water not just polluted but also toxic. On a global scale, millions of people die each year from illnesses caused by the consumption of water contaminated with dangerous microorganisms and toxic pollutants3. The treatment of wastewater has become a real global challenge. The requirement for chemicals, the synthesis of disinfection byproducts, the length of the process, and economics are the limiting factors for the use of different wastewater treatment techniques that have been reported over the last few decades4.
Aquatic ecosystems are being contaminated with dyes used in the textile industry. Approximately, 15% of globally produced dyes end up as toxic effluents and contaminants5. During textile chemical processes, artificial dyes such as azo dyes undergo rapid decomposition and become toxic to the environment due to the formation of aromatic amines because of double bonds in nitrogen6. Thus, effluents discharged from industrial chemical processes result in the coloration of water and the addition of toxic/hazardous substances that are a real threat to aquatic ecosystems7,8. Therefore, International Environmental Standards (IES) have been imposed to create awareness among the public about the threats of effluents released from industry8. Different physicochemical and biological approaches are reported in pertinent literature for the removal of pollutants e.g., dyes from the wastewater9,10. Conventional methods such as flocculation/coagulation, electrochemical and advanced oxidative processes, activated sludge process, reverse osmosis technique, and sorption are thoroughly investigated for the removal of pollutants11,12,13,14,15,16,17. Recently, the photocatalytic degradation technique emerged as a promising approach by using catalysts and light sources. In this technique, the generation of photo-induced OH radicals accelerates the photodegradation of organic effluents into non-toxic chemical species such as water and carbon dioxide without reliance on any separation technique18,19,20,21,22,23. The treated wastewater can be reused in agricultural, chemical, and textile sectors.
These days, nanotechnology is emerging as a powerful interdisciplinary tool and gaining much interest because of its performance and efficacy. Its extraordinary ability to build new atomic structures has already sparked the development of cutting-edge tools and materials with a diverse variety of applications24. Nowadays, multifunctional next-generation nanomaterials and nanocomposites are being synthesized by exploiting the physicochemical characteristics of nanostructures. These physicochemical properties include low density, high adsorptive surface area, more functional groups, differential shapes/orientations, and chemical fitness and suitability25,26. Along with other diverse applications, these nanostructures can treat water/wastewater. Because of their high surface-to-volume ratio, high sensitivity and reactivity, high adsorption capacity, and ease of functionalization, nanomaterials and nanocomposites are particularly well suited for application in wastewater treatment. Different wastewater treatment techniques include adsorption/biosorption, nanofiltration, photocatalysis, disinfection, and sensor technologies27. More recently, the use of nanocomposite photocatalysts is gaining much interest as a cutting-edge technique for the breakdown of pollutants present in wastewater. This technology has shown tremendous potential to treat wastewater since it uses nanocomposite photocatalysts made of doped graphene28.
The superior and wonderful substance known as graphene (G) has a two-dimensional (2D) hexagonal honeycomb lattice structure with a benzene (C6H6) ring. Pristine graphene is referred to be a semi-metal or zero-bandgap material since there is no energy difference between its valence and conduction bands. Graphene can be synthesized by two major routes i.e., bottom-up and top-down routes29. The discovery of graphene has had a profound impact on different scientific, engineering, agricultural, medical, and environmental disciplines, particularly after the investigation of Novoselov et al.30. Graphene is characterized by exceptional traits such as high electro-thermal conductivity, low density, and excellent mechanical, carrier, and optical properties31,32,33. To improve the solubility, conductivity, and physicochemical properties of G, functionalization (covalent or non-covalent) and fabrication of G are done to synthesize modified graphene-based materials such as graphene oxide (GO), reduced GO, G-based derivates, and nanocomposites. These G-based nanomaterials/nanocomposites are superior in morphological and physicochemical characteristics25,26,34,35.
At the advent of the Industrial Revolution, point and non-point releases of industrial effluents and hazardous wastes have increased environmental risks. Both colored and non-colored industrial wastewater effluents have detrimental effects on human health because of the presence of different pollutants. Several methods, such as adsorption onto adsorbents like activated carbon, flocculation, chemical oxidation, ultrafiltration, and clays are used to treat industrial wastewater. Due to the high cost of these technologies, photocatalysis is considered one of the most straightforward and ecologically benign methods for the treatment of wastewater. The key advantage of this approach is that water pollution is broken down using solar energy and a straightforward laboratory setup36.
Recently, a greener approach has been developed to synthesize nanoparticles and nanocomposites by using green chemistry. Conventional methods for synthesizing nanoparticles have a variety of negative effects on the environment and human life. When synthesizing nanoparticles/nanocomposites with traditional methods, toxic/hazardous chemicals and high temperatures are frequently used. To meet this challenge, concepts of green chemistry are manipulated in science and engineering that have resulted in different eco-friendly fields such as green nanotechnology. This approach helps in establishing green processes that are clean, safe, and environment friendly that can replace in practice chemical and physical processes producing nanomaterials and nanocomposites. More recently, green synthesis has been gaining much interest because it is eco-friendly, cost-effective, natural, renewable, and safe with minimal generation of toxic/hazardous substances and uses no or less strong oxidizing or reducing37,38,39,40. Plant-based extracts are enriched with different compounds and substances such as terpenoids and phenolic compounds that can be deployed for the reduction of metallic salts to nanoparticles and can hinder nanoparticle aggregation due to capping or stabilizing characteristics41,42. Azadirachta indica used in the present investigation is enriched in diverse phytochemicals and compounds such as carotenoids, terpenoids, triterpenoid (nimbin), flavonoids, glycosides, alkaloids, salannin, tannin and phenolic substances37,43,44,45. Phytochemicals derived from Azadirachta indica have the potential to sorb on metallic nanoparticle surfaces in addition to their capping property. Furthermore, reducing sugars in the extract of Azadirachta indica is capable of reducing metallic ions and can form metallic nanoparticles46,47,48. Natural products and their derivatives, including wine, different amino acids, glucose, and plant extracts, all include polyphenols, which function as reducing, capping, and stabilizing agents. Similarly, microorganisms including bacteria, yeast, and algae can also be used for the ecologically benign synthesis of nanoparticles49. The present study was conducted to synthesize and characterize iron oxide/reduced graphene oxide (FeNPs/rGO) nanocomposites synthesized via the green approach and tested its application in photocatalytic degradation of methylene blue.
Materials and methods
Fresh leaves of Azadirachta indica were taken from the botanical garden of Forman Christian College University, Lahore (31°32′58.9′′ N 74°20′37.0′′ E). Reagent grade Iron (III) chloride hexahydrate (FeCl3·6H2O), methylene blue, sodium hydroxide (NaOH), graphite powder, concentrated sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), and hydrochloric acid (HCl) were obtained from Sigma-Aldrich and utilized without additional purification.
Preparation of leaf extract
The branches of Azadirachta indica were stripped of their leaves after collection from the botanical garden of Forman Christian College, University, Lahore. To remove the dirt and debris, collected leaves were washed with tap water (once) and distilled water (twice). After washing, the leaves were air-dried for a week in the shade. Using an electronic grinder, dried leaves of Azadirachta indica were ground into a fine powder, which was then sieved through a fine mesh sieve. In a 500 mL beaker, 1.35 g of plant powder and 200 mL of distilled water were added. On a hot plate, stirring was done continuously for two hours in a beaker. The plant extract was filtered using Whatman no. 40 filter paper after two hours of stirring. After filtration, 160 mL of brown-colored plant extract was collected. This was in line with the method reported by Bhuiyan et al.50.
Synthesis of graphene oxide
One gram of graphite powder and 50 mL of concentrated H2SO4 were added to a 500 mL beaker and agitated for about 30 min to synthesize graphene oxide (GO) using a modified Hummer technique. The graphite powder gave the solution its dark color. For one hour, the mixture was cooled to below 5 °C. Six grams of KMnO4 were added to the reaction mixture and then continuously agitated for two hours at a temperature below 15 °C. Then, 90 mL of distilled water was added dropwise to start the oxidation process. After the reaction, the liquid turned dark brown, and the temperature was constantly maintained below 30 °C and stirred for two hours. Then, 280 mL of distilled water and 6 mL of H2O2 were added to terminate the reaction and to remove any extra KMnO4. When the solution was forcefully swirled, a brilliant yellow hue resulted from the reaction. The reaction mixture was filtered before being cleaned with 10% HCl and distilled water. The final product was oven-dried for eight hours at 80 °C. The resultant GO displayed a similar appearance and properties as reported by Ishtiaq et al.51.
Green synthesis of reduced graphene oxide using plant extract
Reduced graphene oxide (rGO) was synthesized via a green approach by using Azadirachta indica plant extract as a capping agent. One gram of synthetic graphene and 5 g of plant extract were combined in a 500 mL beaker, and the mixture was then continuously swirled at 70 °C for an hour to accomplish full reduction. Centrifuged after full reduction, and then rinsed with distilled water and ethanol to achieve pH neutrality following an oven drying process at 80 °C for about 8 h. The method was followed as reported by Anwar et al.52.
Synthesis of magnetite iron nanoparticles
Ferric chloride hexahydrate (FeCl3.6H2O) was used as a precursor material to synthesize magnetite Fe2O3 nanoparticles. At room temperature, a 1:1 mixture of 50 mL of Azadirachta indica plant leaf extract and 50 mL of 0.1 M FeCl3.6H2O solution was added dropwise. Then, 1 M of NaOH was added till the pH reached 11. The production of an intensely black-colored solution after stirring the resulting mixture for about 30 min with a magnetic stirrer demonstrated the synthesis of iron oxide nanoparticles (FeNPs). Centrifugation of the synthesized nanoparticles at 4500 rpm was done for 15 min and washed thrice with ethanol and distilled water and separated these FeNPs. The FeNPs were dried in an oven for 24 h at 65 °C. After drying, magnetite iron nanoparticles were obtained, and their synthesis was in line with Bhuiyan et al.50.
Synthesis of magnetite iron oxides/reduced graphene oxide nanocomposites using Azadirachta indica plant leaf extract
Green deposition was used for producing iron oxide/reduced graphene oxide (FeNPs/rGO) nanocomposites. Iron oxide and reduced graphene oxide were used at a weight ratio of 1:2. Iron oxides (0.08 g) and reduced graphene oxide (0.16 g) were collected individually in 100 mL beakers with 10 mL of distilled water. Both solution combinations were sonicated in an ultrasonicator for 30 min to improve suspension. For three hours, a solution of reduced graphene oxide was stirred continuously on a hot plate while a suspension of iron oxides was fed into it at a rate of around 0.5 mL every ten minutes. Following that, the solution was centrifuged at 4500 rpm for 10 min, and washing was carried out in three phases using distilled water and ethanol to remove any undesirable contaminants. The product was then heated up for 8 h at 65 °C to dry it. The dried product was ground into a fine powder containing magnetite iron oxides and reduced graphene oxide nanocomposite (FeNPs/rGO).
Characterization techniques
For optical evaluations, the absorption spectra of synthesized nanocomposites were analyzed using UV–visible spectroscopy (Cary 50) in the 200–800 nm range. Agilent technology (Cary 630) FT-IR in the 650–4000/cm range was used to investigate functional groups. The crystal phase composition was studied using X-ray diffraction (Bruker D2 Phaser) in the 2θ range 10–80°. The mean particle size of all the synthesized nanomaterials by employing the deby Sherer’s equation. Morphology and elemental analyses were conducted using SEM–EDX (S-3400 N), Hitachi.
Photocatalytic activity
The photocatalytic activity of each sample was assessed against methylene blue (MB) with and without the presence of the catalyst. A 100 mL beaker was filled with 25 ml of a 20 ppm MB solution, which was then stirred for 30 min. After 20 min, 5 mg of nanocomposite was added to this solution and swirled. The absorbance at a certain wavelength (665 nm) was then measured by adding a small quantity of this solution to a cuvette every 5 min. The dye inside the nanocomposite had completely broken down after one hour. The % dye degradation was calculated using the equation suggested by Anwar et al.52.
Ethical approval
The collection of plant materials used in this study complies with relevant institutional, national, and international guidelines and legislation.
Results and discussion
Optical properties
For evaluations of optical properties, absorption spectra of synthesized nanomaterials and nanocomposites were analyzed using UV–visible spectroscopy in the 200–800 nm range. The maximum absorbance of synthesized nanomaterials and nanocomposites i.e., GO, rGO, FeNPs, and FeNPs/rGO was observed at 232, 265, 395, and 405 nm, respectively, confirming the synthesis of graphene-based nanocomposites of magnetite iron oxides with reduced graphene oxide. Absorption peaks between 230 and 240 nm are characteristic peaks of GO confirming the synthesis of GO. The obtained 232 nm absorption peak in the case of GO can be ascribed to π–π* transitions of the remaining sp2 C=C bonds53,54 that was shifted to 265 nm in the case of rGO after reduction of GO by green method using Azadirachta indica leaf extract. During GO reduction, an increase in π conjugation network shifting of absorption towards longer wavelength region is due to less requirement of energy55,56. Similarly, absorption spectra of FeNPs and FeNPs/rGO were observed at 395 and 405 nm, respectively, confirming the synthesis of graphene-based nanoparticles and nanocomposites of magnetite iron oxides with reduced graphene oxide. Band gap values were calculated from UV–visible data and were represented in Fig. 1 using Wood and Tauc plots57.
Fourier transform infrared spectroscopy
Functional groups of GO, rGO, and FeNPs/rGO nanocomposite were observed using FTIR spectrum in the range of 500–4000/cm as shown in Fig. 2a,b. FTIR is a valuable spectroscopic method for characterizing various functional groups, particularly functional groups containing oxygen. The presence of several functional groups including O–H, C–OH, COOH, and C–O in the FTIR spectrum demonstrated that the precursor graphite had been successfully oxidized and GO had been successfully synthesized. A distinctive peak of the stretching mode of O–H functional groups is a large peak of the spectrum between 3500 and 2500 cm−1 58,59,60. The 1573 cm−1 peak is attributed to the stretching of the C=C from the unoxidized domain of graphite, whereas the 1730 cm−1 peak is attributed to the stretching of the carboxyl group58,60. The stretching vibration of C–O from C–O–C is responsible for the 1017 cm−1 peak. The reduction of GO into rGO is visible in the FTIR spectrum of rGO, which is depicted in Fig. 2. This is because the O-containing functionalities' peak intensities are less intense than GO's peak intensities. These results demonstrated the reduction of GO caused by ascorbic acid.
The existence of several functional groups in rGO, however, showed that functional groups are still present in the synthesized material despite its close resemblance to pristine graphene which is in agreement with Andrijanto et al.61.
FTIR spectrum (Fig. 3) showed a characteristic peak at 577 cm−1 which confirmed the synthesis of FeNPs/rGO nanocomposites synthesized by the green deposition method. The absorption band around 577 cm−1 was attributed to FeO (indicating Fe3O4)62 and confirmed the synthesis of FeNPs/rGO nanocomposites. These results are in line with those reported by Sodipo et al.63.
X-ray diffraction analysis
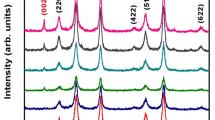
The X-ray diffraction pattern of FeNPs/rGO nanocomposites is shown in Fig. 3a–e. The diffraction peak of rGO was about 2θ = 26° while the diffraction peak of magnetite iron oxide was about 2θ = 44 confirming the synthesis of FeNPs/rGO nanocomposites synthesized via the green deposition method. The diffraction peak of rGO was about 2θ = 26° indicating the production of restacked rGO sheets after reduction of GO64,65. The JCPDS number for metallic iron nanoparticles, like α-Fe (alpha iron), is often 06–0696, reflecting their crystal structure in X-ray diffraction. However, variations can occur based on factors like nanoparticle size, shape, and surface characteristics. Based on Debye Sherrer's equation, the mean particle size of all synthesized materials was calculated, which follows the standard calculation of nanomaterials. As can be seen in Fig. 3a–e, GO nanoparticles have the particle size of (60–80), rGO nanoparticles are (90–95), FeNPs have the particle size of (70–90); Fe/GO nanocomposites have a particle size (40–60) nm, whereas Fe/rGO nanocomposites are of (80–85) nm. Several studies have examined the synthesis and properties of reduced graphene oxide (rGO) and ferric composites, such as Ma66, which presented a controllable rGO/Fe3O4 composite film. Supriya67 investigated the alteration of crystal symmetry in cobalt ferrite-reduced graphene oxide nanocomposites. Studying the synthesis and properties of reduced graphene oxide (rGO) and ferric composites is significant because it can lead to the development of advanced materials with unique properties and applications. These composites have the potential to be used in various fields such as energy storage, catalysis, sensors, and biomedical applications, making them a subject of great interest in scientific research. Sagadevan68 presented a chemically stabilized rGO/ZrO2 nanocomposite synthesis with improved electrical properties. Additionally, Singh69 discussed the outstanding electromagnetic interference shielding capabilities of a lightweight rGO-Fe3O4 nanoparticle composite. As a result of these studies, rGO and ferric composites have been demonstrated to be effective across a wide range of applications, including magnetoelectronics and electromagnetic shielding.
Scanning electron microscopy-energy dispersive X-ray analysis
The morphological and elemental distribution of all the synthesized nanoparticles was verified by scanning electron microscopy attached to the energy dispersive spectroscopy examination. Figure 4a,b reveals the EDX elemental mapping of FeNPs and Fe/rGO composites only respectively. It was observed that the percentage of Fe is 55.61%, carbon is 4.51% and oxygen is 35.72% as shown in Fig. 4a. The binding energies of Fe are related to characteristic peaks around 0.9, 6.1, and 7 keV along with the characteristic peak of oxygen at 0.5 keV. Therefore, the EDX analysis confirmed that both iron & oxygen are present in all the nanocomposites. Results are in agreement with those reported by Rahman et al. and Sayed et al.70,71.
The surface morphology and texture analysis of the synthesized nanocomposites were verified by SEM examination (Fig. 5a–e). Scanning electron microscopy demonstrated the rough surfaces of all the synthesized materials. All types of particles possessed cohesively manifested clustered and spherical shapes. Interactions between graphene-based nanomaterials and magnetite nanoparticles in the nanocomposites are influenced by functional groups and surface chemical properties. Based on the present surface morphologies of the synthesized nanomaterials, it has been established that they can be used for better applications in the removal of dyes and metals from wastewater due to their porous and rough surfaces. These nanomaterials can also be used for the removal of pesticides and other pollutants from contaminated water. These composites of FeNPs are gaining much interest because of their unique optical, electric, and magnetic properties72.
Photocatalytic activity of FeNPs, and Fe/rGO nanocomposite against methylene blue
The percentage degradation of methylene blue (MB) in samples is represented in Fig. 6a,b. The activity was done with FeNPs nanoparticles and with FeNPs/rGO nanocomposites to estimate their relative performance in the degradation of toxic pollutants. The FeNPs alone showed about 16% degradation (Fig. 6a) while when it was combined with reduced graphene oxide to make FeNPs/rGO nanocomposite, then it showed enhanced photocatalytic activity and degradation was about 75% as shown in Fig. 7b. These results agree with those reported by Abid et al.73 and Sadhukhan et al.74.
The first order rate Eq. 2 was used to draw the graph between ln(A − A∞) versus time to conduct the kinetic investigation. Figure 7 illustrated this relationship by stating that the slope gave the value of the first order rate constant, k (min−1), as
The properties and applications of reduced graphene oxide (rGO) and its composites with ferric have been studied in a variety of studies. According to Shahid et al.75 and Iftikhar et al.76, graphene enhances the photocatalytic properties of rGO composites with different ferric materials. An rGO/Fe3O4 composite film was examined by Ma et al.66, demonstrating its potential for a variety of applications. As a demonstration of the versatility of rGO composites, Deepi et al.77 synthesized a novel rGO-SCO nanocomposite with high specific capacitance. The nanocomposite also demonstrated excellent stability in aqueous electrolytes, making it a promising material for energy storage applications. Furthermore, rGO composites have also shown potential for water treatment, corrosion inhibition, and biosensing.
Conclusions
Graphene-based nanomaterials such as graphene oxide (GO), reduced graphene oxide (rGO), iron nanoparticles (FeNPs), and magnetite iron oxide/G-based nanocomposites (FeNPs/rGO) were successfully synthesized using Azadirachta indica leaf extract via green deposition method. Synthesized materials were successfully characterized by UV–vis spectrophotometry, Fourier Transform Infra-Red spectroscopy, XRD, and SEM–EDX techniques. The final synthesized FeNPs/rGO nanocomposites showed remarkable photocatalytic activity in the removal of toxic pollutants such as methylene blue dye from the wastewater. FeNPs/rGO nanocomposites showed 75% degradation against methylene blue which was attributed to its lower band gap. Based on these results, it is plausible to conclude that newly synthesized FeNPs/rGO nanocomposites can also be deployed as a potential photocatalyst degrader for photocatalytic degradation of other different dye pollutants due to lower band gap.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Roccaro, P., Sgroi, M. & Vagliasindi, F. G. A. Removal of xenobiotic compounds from wastewater for environment protection, treatment processes, and costs. Chem. Eng. Trans. 32, 505–510 (2013).
Kanwal, S. et al. Guar gum, Ulva lactuca L. biomass, and xanthan gum-based copolymer novel biosorbent for adsorptive removal of acid orange 10. Biocatal. Agric. Biotech. 58, 103173 (2024).
Cabral, J. P. Water microbiology. Bacterial pathogens and water. Int. J. Environ. Res. Public Health 10, 3657–703 (2010).
Mateo-Sagasta, J., Zadeh, S. M., Turral, H., Burke, J. Water pollution from agriculture: a global review. Executive summary. Rome, Italy: FAO; Colombo, Sri Lanka: International Water Management Institute (IWMI). CGIAR Research Program on Water, Land and Ecosystems (WLE), p. 35. (2017)
Zollinger, H. Color Chemistry: Synthesis, Properties, and Applications of Organic Dyes and Pigments 3rd edn. (Wiley-VCH, Weinheim, Germany, 2003).
Sadhukhan, S. et al. Synthesis of RGO/NiO nanocomposites adopting a green approach and its photocatalytic and antibacterial properties. Mater. Chem. Phys. 247, 122906 (2020).
Aslam, A. et al. Metal oxide impregnated biochar for azo dyes remediation as revealed through kinetics, thermodynamics, and response surface methodology. ACS Omega 9(4), 4300–4316 (2024).
Joshni, T. C. & Subramaniam, K. Enzymatic degradation of azo dyes-a review. Int. J. Environ. Sci. 1, 1250–1260 (2011).
Tarrago, M., Garcia-Valles, M., Aly, M. H. & Martínez, S. Valorization of sludge from a wastewater treatment plant by glass-ceramic production. Ceram. Int. 43, 930–937 (2017).
Niu, J. et al. Effects of environmental factors on sulfamethoxazole photodegradation under simulated sunlight irradiation: kinetics and mechanism. J. Environ. Sci. 2013(25), 1098–1106 (2013).
Kalkan, E., Nadaroğlu, H., Celebi, N. & Tozsin, G. Removal of textile dye Reactive Black 5 from aqueous solution by adsorption on laccase-modified silica fume. Desalin. Water Treat. 52, 6122–6134 (2014).
Abbas, G. et al. Green synthesized silver nanoparticles: Characterization, phytostimulatory impacts, and degradation potential for organic pollutants. Biocatal. Agric. Biotechnol. 55, 102993 (2023).
Carp, O., Huisman, C. L. & Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 32, 33–177 (2004).
Wang, H., Wu, Z., Liu, Y. & Sheng, Z. The characterization of ZnO–anatase–rutile three-component semiconductor and enhanced photocatalytic activity of nitrogen oxides. J. Mol. Catal. Chem. 287, 176–181 (2008).
Tan, B. H., Teng, T. T. & Omar, A. M. Removal of dyes and industrial dye wastes by magnesium chloride. Water Res. 34, 597–601 (2000).
Alexander, K.L., Alt, S., Owens, E., Patel, M., McGovern, L. March. Low fouling reverse osmosis membranes: evidence to the contrary on microfiltered secondary effluent. In Proceedings of the AWWA Membrane Technology Conference, Atlanta, GA, (2003).
Bhattacharyya, A. et al. Development of an auto-phase separable and reusable graphene oxide-potato starch based cross-linked bio-composite adsorbent for removal of methylene blue dye. Int. J. Biol. Macromol. 116, 1037–1048 (2018).
Ali, M. A., Idris, M. R. & Quayum, M. E. Fabrication of ZnO nanoparticles by solution-combustion method for the photocatalytic degradation of organic dye. J. Nanostruct. Chem. 3, 1–6 (2013).
Zhengpeng, W. et al. Preparation, characterization and visible light photocatalytic activity of nitrogen-doped TiO2. J. Wuhan Univ. Technol. –Mater. Sci. 21, 71–78 (2006).
Salavati, H. & Saedi, H. Photocatalytic oxidation of aromatic pollutants and electrochemical behavior in water over nanopolyphosphotungstate supported on In2O3. Int. J. Electrochem. Sci. 10, 4208–4222 (2015).
Elango, G. & Roopan, S. M. Efficacy of SnO2 nanoparticles toward photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 155, 34–38 (2016).
Rao, M. P., Anandan, S., Suresh, S., Asiri, A. M. & Wu, J. J. Surfactant assisted synthesis of copper oxide nanoparticles for photocatalytic degradation of methylene blue in the presence of visible light. J. Energy Environ. Focus 4, 250–255 (2015).
Hayat, K., Gondal, M. A., Khaled, M. M. & Ahmed, S. Effect of operational key parameters on photocatalytic degradation of phenol using nano nickel oxide synthesized by sol-gel method. J. Mol. Catal. Chem. 336, 64–71 (2011).
Oktaviani, O. Nanoparticles: Properties, applications, and toxicities. Jurnal Latihan 1(2), 11–20 (2021).
Neri, G., Fazio, E., Mineo, P. G., Scala, A. & Piperno, A. SERS sensing properties of new graphene/gold nanocomposite. Nanomaterials 9, 1236 (2019).
Cordaro, A., Neri, G., Sciortino, M. T., Scala, A. & Piperno, A. Graphene-based strategies in liquid biopsy and viral diseases diagnosis. Nanomaterials 10, 1014 (2020).
Kumar, S. Smart and innovative nanotechnology applications for water purification. Hybrid Adv. 3, 100044 (2023).
Batool, F. et al. Biosorption potential of Arachis hypogaea derived biochar for Cd and Ni as evidenced through kinetic, isothermal, and thermodynamics modeling. ACS Omega 8(43), 40128–40139 (2023).
Yan, Y. et al. Synthesis of graphene: Potential carbon precursors and approaches. Nanotechol. Rev. 9, 1284–1314 (2012).
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–670 (2004).
Mbayachi, V. B., Ndayiragije, E., Sammani, T., Taj, S. & Mbuta, E. R. Graphene synthesis, characterization and its applications: A review. Res. Chem. 3, 100163 (2021).
Cui, Y., Kundalwal, S. I. & Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 98, 313–333 (2016).
Perreault, F., De Faria, A. F. & Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 44, 5861–5896 (2015).
Siddiqui, S. I. & Chaudhry, S. A. A review on graphene oxide and its composites preparation and their use for the removal of As3+ and As5+ from water under the effect of various parameters: Application of isotherm, kinetic and thermodynamics. Process Saf. Environ. Protect. 119, 138–163 (2018).
Kanwal, S. et al. Adsorption potential of orange rind-based nanosorbents for the removal of cadmium (II) and chromium (VI) from contaminated water. Environ. Sci. Pollut. Res. 30, 110658–110673 (2023).
Alsukaibi, A. K. Various approaches for the detoxification of toxic dyes in wastewater. Processes 10, 1968 (2022).
Pham-Khanh, N. H., Huynh, N. Q., Le, H. N. B. & Ha, T. K. Q. Green synthesis of zinc oxide microparticles using the leaf extract of Dolichandrone spathacea in sustainable agriculture: a new approach for protecting the legume plant (Vigna radiata) against the Cr(VI) stress. Asian J. Agric. Biol. 2024(3), 2023245. https://doi.org/10.35495/ajab.2023.245 (2024).
Alhaithloul, H. A. S. et al. Effect of green-synthesized copper oxide nanoparticles on growth, physiology, nutrient uptake, and cadmium accumulation in Triticum aestivum (L.). Ecotoxicol. Environ. Saf. 268, 115701 (2023).
Hussain, I., Singh, N. B., Singh, A., Singh, H. & Singh, S. C. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 38, 545–560 (2016).
Agharkar, M., Kochrekar, S., Hidouri, S. & Azeez, M. A. Trends in green reduction of graphene oxides, issues and challenges: A review. Mater. Res. Bull. 59, 323–328 (2014).
Younas, M. et al. Synthesis and characterization of cerium, silver and copper oxide nanoparticles and their anticancer potential of hepatocellular carcinoma HepG2 cancer cells. J. Mol. Struct. 1288, 135756 (2023).
Kanagasubbulakshmi, S. & Kadirvelu, K. Green synthesis of iron oxide nanoparticles using Lagenaria siceraria and evaluation of its antimicrobial activity. Def. Life Sci. J. 2, 422–427 (2017).
Masood, N. et al. Green synthesis, characterization and adsorption of chromium and cadmium from wastewater using cerium oxide nanoparticles; reaction kinetics study. J. Mol. Struct. 1294, 136563 (2023).
Biswas, K., Chattopadhyay, I., Banerjee, R. K. & Bandyopadhyay, U. Biological activities and medicinal properties of Neem (Azadirachta indica). Curr. Sci. 82, 1336–1345 (2002).
Hareesh, K. et al. Bio-green synthesis of Ag–GO, Au–GO and Ag–Au–GO nanocomposites using Azadirachta indica: its application in SERS and cell viability. Mater. Res. Express 3, 075010 (2016).
Shankar, S. S., Rai, A., Ahmad, A. & Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid. Interface Sci. 275, 496–502 (2004).
Bindhani, B. K. & Panigrahi, A. K. Green synthesis of gold nanoparticles using neem (Azadirachta indica L.) leaf extract and its biomedical applications. Int. J. Adv. Biotechnol. Res. 5, 457–64 (2014).
Rasool, M., Rasool, M. H., Khurshid, M. & Aslam, B. Biogenic Synthesis and Characterization of silver nanoparticles: exploring antioxidant and anti-inflammatory activities and assessing antimicrobial potential against multidrug-resistant bacteria. Asian J. Agric. Biol. 2024(3), 2023364. https://doi.org/10.35495/ajab.2023.364 (2024).
Ahmed, S. F. et al. Green approaches in synthesizing nanomaterials for environmental nanobioremediation: Technological advancements, applications, benefits and challenges. Environ. Res. 204, 111967 (2022).
Bhuiyan, M. S. et al. Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 6, e04603 (2020).
Ishtiaq, A., Farrukh, M. A., Rehman, A. U., Karim, S. & Chong, K. K. Facile synthesis of zwitterionic surfactant-assisted molybdenum oxide/reduced graphene oxide nanocomposite with enhanced photocatalytic and antimicrobial activities. J Chin. Chem. Soc. 69, 269–279 (2022).
Anwar, A. W. et al. Simple and inexpensive synthesis of rGO-(Ag, Ni) nanocomposites via green methods. Mater. Technol. 30, 155–160 (2015).
Clark, B. J., Frost, T. & Russell, M. A. UV Spectroscopy: Techniques, instrumentation and data handling (Springer Science & Business Media, New York, 1993).
Mei, Q. et al. Highly efficient photoluminescent graphene oxide with tunable surface properties. Chem. Commun. 46, 7319–7321 (2010).
Li, D., Müller, M. B., Gilje, S., Kaner, R. B. & Wallace, G. G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 3, 101–105 (2008).
Zboril, R. & Kim, K. S. Functionalization of graphene, covalent and noncovalent approaches, derivatives and applications. Chem. Rev. 112, 6156–6214 (2012).
Atul Kumar, M., Sharma, A., Maurya, I. K., Thakur, A. & Kumar, S. Synthesis of ultra-small iron oxide and doped iron oxide nanostructures and their antimicrobial activities. J. Taibah Univ. Sci. 13, 280–285 (2019).
Shahriary, L. & Athawale, A. A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng 2, 58–63 (2014).
Zhang, T. Y. & Zhang, D. Aqueous colloids of graphene oxide nanosheets by exfoliation of graphite oxide without ultrasonication. Bull. Mater. Sci. 34, 25–28 (2011).
Guo, H. L., Wang, X. F., Qian, Q. Y., Wang, F. B. & Xia, X. H. A green approach to the synthesis of graphene nanosheets. ACS Nano 3, 2653–2659 (2009).
Andrijanto, E., Shoelarta, S., Subiyanto, G. & Rifki, S. Facile synthesis of graphene from graphite using ascorbic acid as reducing agent. AIP Conf. Proc. 1725, 020003 (2016).
Chang, Y. P., Ren, C. L., Qu, J. & Chen, X. G. Preparation and characterization of Fe3O4/graphene nanocomposite and investigation of its adsorption performance for aniline and p-chloroaniline. Appl. Surf. Sci. 261, 504–509 (2012).
Sodipo, B. K. & Azlan, A. A. Superparamagnetic iron oxide nanoparticles incorporated into silica nanoparticles by inelastic collision via ultrasonic field: Role of colloidal stability. AIP Conf. Proc. 1657, 100002 (2015).
Johra, F. T., Lee, J. W. & Jung, W. G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 20, 2883–2887 (2014).
Ali, I. et al. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. Res. 25, 7315–7329 (2018).
Ma, C., Yang, K., Wang, L. & Wang, X. Facile synthesis of reduced graphene oxide/Fe3O4 nanocomposite film. J. Appl. Biomater. Funct. Mater. 15, 1–6 (2017).
Supriya, S., Kumar, S. & Kar, M. Impedance spectroscopy studies in cobalt ferrite-reduced graphene oxide nanocomposite. AIC Conf. Proc. 1728, 020566 (2016).
Suresh, S., Chowdhury, Z., Md, E. & Podder, J. Chemically stabilized reduced graphene oxide/zirconia nanocomposite: Synthesis and characterization. Mater Res Express 4(11), 115031 (2017).
Singh, A. K., Kumar, A., Haldar, K. K., Gupta, V. & Singh, K. Lightweight reduced graphene oxide-Fe3O4 nanoparticle composite in the quest for an excellent electromagnetic interference shielding material. Nanotechnology 29(24), 245203 (2018).
Rahman, S. S. U. et al. Single-step growth of iron oxide nanoparticles and their use as glucose biosensor. Res. Phys. 7, 4451–4456 (2017).
Sayed, F. N. & Polshettiwar, V. Facile and sustainable synthesis of shaped iron oxide nanoparticles: Effect of iron precursor salts on the shapes of iron oxides. Sci. Rep. 5, 9733 (2015).
Wan, J., Cai, W., Feng, J., Meng, X. & Liu, E. In situ decoration of carbon nanotubes with nearly monodisperse magnetite nanoparticles in liquid polyols. J. Mater. Chem. 17, 1188–1192 (2007).
Abid, M. A., Abid, D. A., Aziz, W. J. & Rashid, T. M. Iron oxide nanoparticles synthesized using garlic and onion peel extracts rapidly degrade methylene blue dye. Phys. B: Cond. Matter 622, 413277 (2021).
Sadhukhan, S. et al. Synthesis of RGO/NiO nanocomposites adopting a green approach and its photocatalytic and antibacterial properties. Mater. Chem. Phys. 2020(247), 122906 (2020).
Shahid, M. et al. Dysprosium substituted nickel cobalt ferrite nanomaterials and their composites with reduced graphene oxide for photocatalysis. J. Taibah Univ. Sci. 14(1), 1308–1316 (2020).
Iftikhar, A. et al. Erbium-substituted Ni0.4Co0.6Fe2O4 ferrite nanoparticles and their hybrids with reduced graphene oxide as magnetically separable powder photocatalyst. Ceram. Int. 46(1), 1203–1210 (2020).
Deepi, A., Srikesh, G. & Nesaraj, A. S. One pot reflux synthesis of reduced graphene oxide decorated with silver/cobalt oxide: A novel nanocomposite material for high capacitance applications. Ceram. Int. 44(16), 20524–20530 (2018).
Acknowledgements
The authors extend their appreciation to the Researchers supporting project number (RSP2024R469), King Saud University, Riyadh, Saudi Arabia. The authors greatly acknowledge the financial support by HEC, Pakistan to pursue this research work. The authors also acknowledge the support of NRF and MIST, Korea.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research is funded by the Higher Education Commission (HEC) Pakistan through the NRPU Project (No. 20-16094/NRPU/R&D/HEC/2021 2021). The authors extend their appreciation to the Researchers supporting project number (RSP2024R469), King Saud University, Riyadh, Saudi Arabia. This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (No.NRF-2022K1A3A9A05036564).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N.; Data curation, M.S.A., and S.F.; Formal analysis, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N.; Funding acquisition, M.S.A., M.R., and A.M.A.; Investigation, M.S.A., and S.F.; Methodology, M.S.A., S.A., M.V., S.C., A.D., A.M. A., and R.I.; Project administration, M.S.A., M.R., and A.D.; Resources, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N.; Software, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N.; Supervision, M.S.A.; Validation, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N.; Visualization, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N.; Writing—original draft, M.S.A., and S.F.; Writing—review & editing, M.S.A., S.F., S.A., S.C., A.D., M.A.I., A.M.A., R.I., W.A.A., M.R., and Y.N. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Akhtar, M.S., Fiaz, S., Aslam, S. et al. Green synthesis of magnetite iron oxide nanoparticles using Azadirachta indica leaf extract loaded on reduced graphene oxide and degradation of methylene blue. Sci Rep 14, 18172 (2024). https://doi.org/10.1038/s41598-024-69184-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69184-y
- Springer Nature Limited