Abstract
Bone marrow mesenchymal stem cell-derived exosomes (BMSC-Exos) have been shown to promote angiogenesis after ischemic stroke, in which microRNAs (miRs) are believed to play an important role in exosome-mediated therapeutic effects, though the mechanism is still not clear. In this study, a series of molecular biological and cellular assays, both in vitro and in vivo, were performed to elucidate the role of exosomal miR-486 in angiogenesis following cerebral ischemic and its molecular mechanisms. Our results revealed that BMSC-Exos significantly improved neurological function and increased microvessel density in ischemic stroke rats. In vitro assays showed that BMSC-Exos promoted the proliferation, migration, and tube formation ability of oxygen–glucose deprivation/reoxygenation (OGD/R) injured rat brain microvascular endothelial cells (RBMECs). Importantly, BMSC-Exos increased the expression of miR-486 and phosphorylated protein kinase B (p-Akt) and down-regulated the protein level of phosphatase and tensin homolog (PTEN) in vivo and in vitro. Mechanistic studies demonstrated that transfection with miR-486 mimic enhanced RBMECs angiogenesis and increased p-Akt expression, while inhibited PTEN expression. On the other hand, the miR-486 inhibitor induced an opposite effect, which could be blocked by PTEN siRNA. It was thus concluded that exosomal miR-486 from BMSCs may enhance the functional recovery by promoting angiogenesis following cerebral ischemic injury, which might be related to its regulation of the PTEN/Akt pathway.
Similar content being viewed by others
Introduction
Ischemic stroke (IS) is a condition caused by the abrupt cut-off of blood flow in a specific brain region, leading to the necrosis of local brain tissue. Due to the complex and multistep pathophysiological processes involved in ischemic brain injury, currently available treatments are limited1. The stem cell therapy in recent years has emerged as a promising approach for the regeneration and repair of damaged brain tissue2. Among the different types of cells used in stem cell therapy, bone marrow mesenchymal stem cells (BMSCs) have been extensively studied as a safe and effective treatment for ischemic stroke3,4,5. Despite the wide reporting of BMSCs transplantation’s protective effect on brain injury, the underlying mechanism by which BMSCs promote injury repair after cerebral infarction remains unknown.
Growing evidence suggests that stem cell-mediated neural repair by secreting trophic factors and extracellular vesicles (EVs), which direct a range of restorative processes, including angiogenesis, neurogenesis, axon regeneration, and synaptogenesis6,7. Among the EVs, exosomes are small, measuring 30–150 nm in diameter, and packed with biological components. These components include proteins, lipids, mRNA, and microRNAs (miRNAs, miRs) that can be transferred to target cells and trigger downstream signaling pathways8,9. Stem cell-derived exosomes are the most powerful paracrine components for intercellular communication and have significant therapeutic potential for repairing damaged tissue10. Recent studies indicate that exosomes derived from bone marrow mesenchymal stem cells (BMSC-Exos) can transfer miRs to recipient cells in the brain, which then help repair both the nervous and vascular systems, ultimately alleviating cerebral ischemic injury11,12,13.
Previous studies have shown that increased microvessel density in the peri-infarct region after ischemic stroke is closely associated with longer survival time and long-term functional recovery14,15. Therefore, therapeutic angiogenesis is a crucial therapeutic target for promoting neural repair and neurological functional recovery after ischemic stroke16,17. A growing body of literature reports that exosomes from mesenchymal stem cells (MSCs) promote angiogenesis and improve tissue damage by transporting miRs18,19,20. Recently, we found that BMSC-Exos facilitated angiogenesis and ameliorated brain injury in ischemic stroke mice by delivering miR-21-5p21.
MiR-486, which was found to be abundant in BMSC-Exos through small RNA-sequencing22, has been shown in pre-clinical studies to be closely linked to biological processes, such as cell proliferation, migration, angiogenesis, and apoptosis23. Li et al. reported that miR-486 from mesenchymal stem cell-derived exosomes effectively promoted angiogenesis and cardiac recovery in mice and nonhuman primates with myocardial infarction24. Similarly, Lu et al. found that miR-486 transferred by exosomes from adipose-derived stem cells enhanced wound healing and angiogenesis of human microvascular endothelial cells25. However, the effect and mechanism of BMSC-derived exosomal miR-486 on cerebral ischemic injury's angiogenesis have not yet been thoroughly researched.
The present study was aimed to investigate the pro-angiogenic effects of BMSC-Exos in ischemic stroke rats, and to further elucidate the underlying mechanism that BMSC-derived exosomal miR-486 protects rat brain microvascular endothelial cells (RBMECs) against oxygen and glucose deprivation and reperfusion (OGD/R)-induced injury in vitro. Our findings provide novel insights into the potential therapeutic use of BMSC-Exos for cerebral ischemia and shed light on the role of miR-486 in mediating the beneficial effects of BMSC-Exos.
Materials and methods
Animals
Male Sprague–Dawley rats were obtained from SIPPR/BK Laboratory Animals (Shanghai, China) and housed in a specific pathogen free (SPF) animal facility with free access to water and food. Rats weighting between 80–100 g (4 weeks old) and 280–300 g (12 weeks old) were utilized for the isolation of BMSCs and the induction of the middle cerebral artery occlusion (MCAO) model, respectively. The SPF animal facility was maintained at a controlled temperature (22 ± 2 °C) and humidity (60 ± 5%), with a 12 h light–dark cycle. The animal study was reviewed and approved by the Experimental Animal Care and Ethics Committee of Zhejiang Chinese Medical University (Reference number: IACUC-20181126-13). All authors confirm that all animal procedures and care were performed in accordance with relevant guidelines and regulations. All animal experiments were performed in accordance with the ARRIVE guidelines.
BMSCs culture
BMSCs were isolated and cultured using a previously described method26. Briefly, bone marrow was extracted from the femurs and tibias of rats and rinsed with precooled Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12, Gino Bio-Pharm Technology, Zhejiang, China) containing 1% penicillin and streptomycin (Haotian Biological Technology, Zhejiang, China). The bone marrow was then disrupted and digested to obtain a single-cell suspension, which was filtered and cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS, GEMINI, CA, USA). The medium was refreshed 48 h later, and then every three days thereafter. Upon reaching 80–90% confluency, the cells were subcultured at a 1:2 ratio to maintain their growth and viability. For subsequent experiments, BMSCs at passages 3–5 were selected to ensure uniformity and consistency of the cell phenotype and function.
RBMECs culture
RBMECs were obtained from the American Type Culture Collection and cultured in DMEM (Gibco, New York, USA) media, containing 10% FBS, 1% penicillin and streptomycin, at a temperature of 37 °C and with 5% CO2. The cells were routinely passaged every two to three days.
Cell transfection
MiR-486 mimic/negative control (NC), miR-486 inhibitor/NC, and PTEN siRNA/NC were synthesized by RiboBio (Supplementary Table 1, Guangzhou Ribobio, Guangzhou, China). Transfection of the miR-486 mimic/inhibitor or PTEN siRNA was performed using Lipofectamine 2000 reagent (Invitrogen, CA, USA) and Opti-MEM medium (Invitrogen, CA, USA) when cells reached 80–90% confluence. After 6 h, the medium was replaced with exosome-free FBS-containing medium (10%).
Exosomes extraction and identification
Using differential ultracentrifugation, the BMSC-Exos were extracted and purified. The supernatants of BMSCs were collected after 48 h of incubation in a medium containing 10% exosome-free FBS, and sequentially centrifuged at 300 × g, 2000 × g, and 10,000 × g for 10, 10, and 30 min respectively. The supernatant was then filtered through a 0.22 μm microporous membrane and transferred to an ultra-isolation chamber, where it was subjected to ultracentrifugation at 100,000 × g for 2 h at 4 °C. The resulting sediment was resuspended in 100 μL PBS, and overall protein levels were assessed using a Micro BCA Protein Assay kit (Thermo Scientific, Rockford, IL, USA). The samples were then stored at − 80 °C. To identify exosomes, transmission electron microscopy (TEM, Hitachi, Japan), nanoparticle tracking analysis (NTA, Particle Metrix, Germany), and Western blot were used, following procedures outlined in a previous study21.
Rat middle cerebral artery occlusion (MCAO) model establishment
The MCAO model was employed as per the established protocol27. Initially, the rats were administered with isoflurane anesthesia and then positioned in a supine posture on the operating table. Next, the right common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were meticulously separated. Subsequently, insertion of a 4–0 nylon suture was carried out through the ECA and progressed into the ICA till approximately 18 ± 2 mm from the bifurcation. The rats’ rectal temperature was maintained at approximately 37 °C throughout the entire period of 90 min of ischemia. The sham-operated rats underwent an identical procedure, but the insertion of the nylon suture was not performed.
BMSC-Exos administration and BrdU labeling
To evaluate the therapeutic effects of BMSC-Exos, rats were divided into three groups: sham group, MCAO group and BMSC-Exos group. In the BMSC-Exos group, 100 μg of BMSC-Exos per rat was dissolved in 0.5 mL PBS and was administered via tail vein injection 24 h post-reperfusion, while the Sham group and MCAO group received an equivalent volume of PBS. Subsequently, BrdU was intraperitoneally injected 24 h post-reperfusion, at a dose of 50 mg/kg/d for 14 consecutive days.
Neurological function evaluation
Neurological deficit assessments were conducted prior to inducing MCAO and post-surgery, on the 3rd, 7th, and 14th days. The extent of sensorimotor deficits was evaluated using the modified neurological severity score (mNSS), which measures a score range of 0 to 18 (0 signifies a normal condition, and 18 represents maximal deficit)28. Sensorimotor function was assessed using the corner test, in which each rat was subjected to 10 trials, and the number of right turns was recorded29.
Immunofluorescence staining
The double immunofluorescence staining was employed to detect BrdU/von Willebrand factor (vWF) utilizing the methods reported in a prior study21. The rats were sacrificed 14 days following MCAO, and their brains were cut into 10 μm-thick frozen sections (Leica, Wetzlar, Germany). After blocked with 5% goat serum for 1 h, brain sections thereafter incubated with anti-BrdU and anti-vWF primary antibodies (1:200, Sigma, MO, USA and 1:100, Abcam, MA, USA, respectively) overnight at 4 °C. Secondary antibodies were then employed at 37 °C for 1 h, prior to counterstaining with 4ʹ, 6-diamidino-2-phenylindole (DAPI; Zhongshan Golden Bridge Biotechnology, Beijing, China). Analysis of positive cells was conducted by counting in three random fields.
BMSC-Exos uptake by RBMECs
The exosomes (10 μg) were labeled with PKH67 (2 µL, Sigma-Aldrich, Missouri, USA) and incubated with RBMECs in DMEM supplemented with 5% FBS at 37 °C for 12 h. After washing with PBS, the labeled RBMECs were fixed with 4% paraformaldehyde for 15 min. Subsequently, they were treated with 0.3% TritonX-100 for 30 min, followed by staining with 200 µL DAPI solution (Southern Biotech, Alabama, USA) for 5 min. The samples were then observed under a fluorescence microscope to visualize the fluorescence.
BMSC-Exos treatment and oxygen and glucose deprivation/reoxygenation (OGD/R) model establishment
Prior to OGD/R injury, RBMECs were pretreated with a dose of 100 μg/mL of BMSC-Exos for 16 h. The cells were then cultured with sugar-free DMEM and exposed to hypoxic conditions (5% CO2, 95% N2) for 4, 6, 8, 10, or 12 h to induce OGD, followed by switching to and keeping at normal oxygen level for 12 h of reoxygenation.
Cell viability measurement
To perform the OGD/R model, RBMECs were seeded onto 96-well plates at a density of 1 × 104 cells/well and allowed to reach 80% confluence, followed by 12-h of reoxygenation. Ten microliters of Cell Counting Kit-8 solution (CCK-8, Beyotime, Shanghai, China) was then added to each well, and the optical density (OD) after 2 h was read with a microplate reader (Tecan Austria GmbH, Grodig, Austria). For each group, the reading of OD value was repeated five times.
Scratch wound healing assay
RBMECs were seeded onto six-well plates at a density of 4 × 105 cells/well. Following OGD/R treatment, the cells were scratched with a 200 μL pipette tip. The extent of the injuries was photographed at 0, 24, and 48 h, respectively. The migration rate was calculated using the following formula: migration rate (%) = [(initial wound area (t = 0 h) − residual area (t = 24/48 h))/initial wound area (t = 0 h)] × 100%. The assay of each group was repeated triple times.
Transwell migration assay
Cell migration was measured using Transwell cell chambers (8 μm, Corning, NY, USA). After OGD/R treatment, RBMECs at a density of 6 × 104 cells/well were suspended in 100 μL of FBS-free DMEM and seeded into the upper chamber. In the lower chamber, 500 μL of DMEM containing 10% FBS was added. After 24 h of migration, cells were fixed with 4% paraformaldehyde solution and stained with 0.1% crystal violet (Sangon Biotech, Shanghai, China). Each group was performed in triplicate.
Tube formation assay
To initiate tube formation, RBMECs (6 × 104 cells/well) were seeded in FBS-free DMEM onto a Matrigel-coated 48-well plate (Corning, MA, USA) following OGD/R. Tubular structures were observed after a 6-h incubation period. Tube length and branch points were measured at five random fields using Image-Pro Plus 6.0 software.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
First, total RNA was extracted and reverse transcribed into complementary deoxyribonucleic acid (cDNA) using the Mir-X miRNA First-Strand Synthesis Kit (TaKaRa, Liaoning, China). Subsequently, for the PCR reactions, the cDNA was amplified using the SYBR Green Ex Taq Kit (TaKaRa, Liaoning, China). The specific 5ʹ primer sequence for miR-486 was 5ʹ-TCCTGTACTGAGCTGCCCC-3ʹ, and the universal 3ʹ primer was mRQ 3ʹ Primer supplied with the Mir-X miRNA First-Strand Synthesis Kit. The expression level of miR-486 was determined using the 2−ΔΔCt method and normalized to U6. Each experiment included at least three biological replicates with three technical replicates per sample from different groups.
Western blot analysis
Total protein was extracted using RIPA lysis buffer (Beyotime, Shanghai, China), and protein concentration was determined using a BCA kit (Beyotime, Shanghai, China). Samples were separated on a 10% SDS-PAGE (Beyotime, Shanghai, China). Membranes were incubated overnight at 4 °C with primary antibodies: rabbit anti-CD9 (1:1000; Bioworld, MN, USA), anti-CD63 (1:1000; Bioworld, MN, USA), anti-ALIX (1:1000; Abcam, MA, USA), anti-PTEN (1:1000; Cell Signaling Technology, MA, USA), anti-p-Akt (1:1000; Cell Signaling Technology, MA, USA), anti-Akt (1:1000; Cell Signaling Technology, MA, USA), and a mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1000, Hua Biotechnology, Zhejiang, China). On the following day, membranes were incubated with corresponding secondary antibodies: goat anti-rabbit IgG (1:1000; Broker Biological Technology, Zhejiang, China), and goat anti-mouse IgG (1:1000; Bioker Biological Technology, Zhejiang, China). Protein band visualization was performed using an enhanced chemiluminescence detection kit (Millipore, MA, USA). Original images are shown in Supplementary Figs. 1–4.
Data processing and statistics
Data analysis was performed using SPSS 25.0 software (Chicago, IL, USA) and presented as mean ± standard deviation (SD). Neurological deficit and corner test data were analyzed by nonparametric Kruskal–Wallis H test. One-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls posthoc test was used to analyze all other data. A p-value of less than 0.05 was considered to be statistically significant.
Ethics statement
The animal study was reviewed and approved by the Experimental Animal Care and Ethics Committee of Zhejiang Chinese Medical University (Reference number: IACUC-20181126-13). All authors confirm that all animal procedures and care were performed in accordance with relevant guidelines and regulations. All animal experiments were performed in accordance with the ARRIVE guidelines.
Results
BMSC-Exos identification
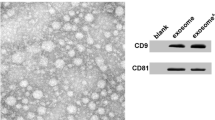
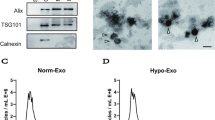
The identification of BMSC-Exos was carried out using previously described methods21. TEM results showed that BMSC-Exos were cup-shaped vesicles (Fig. 1A). Nanoparticle tracking analysis (NTA) revealed that the BMSC-Exos had an average size of 123.2 ± 3.7 nm, with concentration of approximately 2.04 × 1010 particles/mL (Fig. 1B). The presence of specific exosome surface markers, including ALIX, CD63, and CD9, was confirmed in BMSC-Exos (Fig. 1C). Collectively, these findings suggested that the isolated particles be indeed exosomes derived from BMSCs.
BMSC-Exos improved neurological function recovery after MCAO
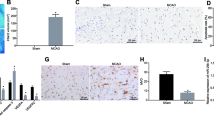
To study the long-term neurological function recovery generated by BMSC-Exos, we conducted mNSS and corner test on days 1, 3, 7, and 14 after MCAO. The results, depicted in Fig. 2A,B, showed that the BMSC-Exos group demonstrated significant improvement in both mNSS and corner test compared to that of the MCAO group at 7 and 14 days, respectively.
BMSC-Exos improved behavioral outcomes and promoted angiogenesis in rats after MCAO. (A) Neurological deficit scores. (B) Corner test. #p < 0.05 compared with Sham group. *p < 0.05 compared with MCAO group. n = 12. (C) Representative immunofluorescence images of the rats peri-infarct zone. Scale bar = 100 μm. (D) Quantification of vWF+/DAPI+ cells. n = 6. (E) Quantification of vWF+/BrdU+/DAPI+ cells. n = 6. (F) The expression level of miR-486 in the peri-infarct cortex of MCAO rats. n = 3. (G–I) Representative western blot images and quantification of densitometries of Western blot band. n = 3. The data are presented as the mean ± SD. #p < 0.05, ##p < 0.01 compared with Sham group. *p < 0.05, **p < 0.01 compared with MCAO group.
BMSC-Exos promoted angiogenesis and miR-486 expression in the peri-infarct cortex of MCAO rats
On day 14 after MCAO, we measured the angiogenic effect of BMSC-Exos using BrdU/vWF immunofluorescent staining. The results presented in Fig. 2C–E reveal an elevation in the number of vWF+ cells within the peri-infarct cortex of both the MCAO and BMSC-Exos groups when compared to sham group. Crucially, the BMSC-Exos groups exhibited a significant increase in the number of BrdU+/vWF+ cells compared to the MCAO group, indicating a pronounced angiogenic effect. In addition, we evaluated the expression of miR-486 in the ischemic boundary zone using qRT-PCR on day 14 post-MCAO. Our findings, as shown in Fig. 2F, indicated that the administration of BMSC-Exos increased the expression of miR-486.
BMSC-Exos decreased PTEN expression and increased p-Akt/Akt expression in the peri-infarct cortex of MCAO rats
To test whether BMSC-Exos promotes angiogenesis through PTEN/Akt pathway, the protein level of PTEN and p-Akt/Akt were detected by western blot analysis. As shown in Fig. 2G–I, the protein level of PTEN was upregulated in MCAO group compared to that in the sham group, while the protein level of p-Akt/Akt was decreased. More importantly, BMSC-Exos reversed the altered levels of these above proteins.
BMSC-Exos uptaken by RBMECs
To study the uptake of BMSC-Exos into RBEMCs, we labeled BMSC-Exos with PKH67 dye. We then incubated the labeled BMSC-Exos with RBMECs for 12 h, and Fig. 3A illustrates the subsequent endocytosis of PKH67-labeled BMSC-Exos by RBMECs.
BMSC-Exos promoted the proliferation, migration, and tube formation of OGD/R-treated RBMECs. (A) PKH67-labeled BMSC-Exos were uptake by RBMECs. Scale bar = 100 μm. n = 3. (B) Cell viability of RBMECs treated with OGD/R. n = 5. (C) Cell viability of RBMECs treated with BMSC-Exos at concentrations 0, 10, 25, 25, 50, 100, and 200 μg/mL. n = 5. (D–F) Representative images and quantification of scratch assay of RBMECs. Scale bar = 200 μm. n = 3. (G, I) Representative images and quantification of transwell assay of RBMECs. Scale bar = 100 μm. n = 3. (H, J, K) Representative images and quantification of tube formation ability of RBMECs. Scale bar = 100 μm. n = 3. The data are presented as the mean ± SD. *p < 0.05, **p < 0.01.
BMSC-Exos promoted the OGD/R-treated RBMECs angiogenesis
We assessed the cell viability of RBMECs exposed to OGD/R using the CCK-8 assay. Our experimental results demonstrated that the cell viability of RBMECs decreased progressively with the prolonged duration of OGD. Compared to the control group, the cell viability of RBMECs exposed to OGD for 8 h followed by reoxygenation for 12 h decreased by approximately 40%. Consequently, we selected the OGD 8 h and reoxygenation 12 h for subsequent experiments, as illustrated in Fig. 3B. We also observed that the protective effect of BMSC-Exos on OGD/R-treated RBMECs increased gradually in the range of 50–200 µg/mL, with the optimal effect observed at 100 µg/mL exosomes (Fig. 3C). This value was then selected for subsequent mechanism research.
Thereafter, we investigated the impact of BMSC-Exos on the proliferation, migration, and tube formation of OGD/R injured RBMECs, as these processes were crucial in angiogenesis. Our results in Fig. 3D–K suggest that BMSC-Exos possess the ability to improve the migration and tube formation capabilities of OGD/R-treated RBMECs. These findings indicate that BMSC-Exos promotes the angiogenesis of RBMECs after OGD/R injury.
BMSC-Exos increased miR-486 expression in OGD/R-treated RBMECs by transferring miR-486
To confirm that BMSC-Exos by transferring miR-486 increased the expression of miR-486 in OGD/R-treated RBMECs, the expression of miR-486 was detected by qRT-PCR, which showed significantly lower expression of miR-486 in the OGD/R group as compared to that of the control group. However, after pretreatment with BMSC-Exos, the expression of miR-486 was significantly increased (Fig. 4A). Furthermore, transfecting BMSCs with miR-486 mimic or inhibitor, respectively led to subsequent increase or decrease of the expression of miR-486 in BMSCs and BMSC-Exos correspondingly (Fig. 4B,C). Finally, OGD/R-treated RBMECs were dealed with exosomes derived from BMSCs transfection with miR-486 mimic (miR-486 mimic-Exos) or inhibitor (miR-486 inhibitor-Exos). The results showed that miR-486 mimic-Exos significantly increased the miR-486 expression in OGD/R-treated RBMECs, while miR-486 inhibitor-Exos inversely affected miR-486 level (Fig. 4D). Taken together, these findings suggest that BMSC-Exos increase the miR-486 expression in OGD/R-treated RBMECs via transferring miR-486.
BMSC-Exos increased miR-486 expression in RBMECs after OGD/R. (A) miR-486 expression levels in the OGD/R-treated RBMECs. (B) miR-486 expression levels in the BMSCs after miR-486 mimic/inhibitor transfection. (C) miR-486 expression levels in the BMSC-Exos after miR-486 mimic/inhibitor transfection. (D) miR-486 expression levels in the OGD/R-treated RBMECs after co-cultured with miR-486 mimic/inhibitor-Exos. The data are presented as the mean ± SD. n = 3. **p < 0.01.
BMSC-Exos promoted OGD/R-treated RBMECs angiogenesis by transferring miR-486
To further explore whether BMSC-Exos promote angiogenesis of OGD/R-treated RBMECs through transporting miR-486, we transfected BMSCs with either miR-486 mimic or inhibitor. Then, we looked at the angiogenic effects of miR-486 mimic-Exos and miR-486 inhibitor-Exos. According to the experimental findings concerning the proliferation (Fig. 5A), migration (Fig. 5B–G), and tube formation capabilities (Fig. 5F,H,I) of RBMECs, miR-486 mimic-Exos markedly augments the pro-angiogenic effects, whereas miR-486 inhibitor-Exos elicited an opposing effect. Hence, our data suggest that BMSC-Exos exert pro-angiogenic activities, at least in part, by transferring miR-486 to recipient RBMECs.
BMSC-Exos promoted RBMECs angiogenesis by transferring miR-486. (A) Cell viability of RBMECs co-cultured with miR-486 mimic/inhibitor-Exos. n = 5. (B–D) Representative images and quantification of scratch assay of RBMECs. Scale bar = 200 μm. n = 3. (E, G) Representative images and quantification of transwell assay of RBMECs. Scale bar = 100 μm. n = 3. (F, H, I) Representative images and quantification of tube formation ability of RBMECs. Scale bar = 100 μm. n = 3. The data are presented as the mean ± SD. **p < 0.01.
Exosomal miR-486 reduced the protein expression level of PTEN in OGD/R-treated RBMECs
Previous studies have demonstrated that PTEN is among the downstream targets of miR-48630. Our western blot results (Fig. 6A) revealed that the overexpression of miR-486 significantly reduced the protein expression of PTEN compared to the control group. Conversely, transfection with miR-486 inhibitor resulted in a significant increase in PTEN protein expression in RBMECs. Next, we treated OGD/R-injured RBMECs with miR-486 mimic-Exos or miR-486 inhibitor-Exos. Our data indicated that BMSC-Exos treatment significantly decreased PTEN expression in OGD/R-injured RBMECs, miR-486 mimic-Exos further suppressed PTEN expression, while miR-486 inhibitor-Exos increased PTEN expression (Fig. 6B). Taken together, these findings indicated that BMSCs-derived exosomal miR-486 reduced the protein expression level of PTEN in OGD/R-treated RBMECs, suggesting that PTEN may be a target gene of miR-486.
BMSCs exosomal miR-486 down-regulated the protein expression level of PTEN in RBMECs. (A) Representative western blot images of PTEN in RBMECs transfected with miR-486 mimic/inhibitor and quantification of densitometries of Western blot band. (B) Representative western blot images of PTEN in the OGD/R-treated RBMECs treatment with miR-486 mimic/inhibitor-Exos and quantification of densitometries of Western blot band. C Representative western blot images of PTEN in RBMECs transfected with PTEN siRNA and quantification of densitometries of Western blot band. The data are presented as the mean ± SD. n = 3. *p < 0.05, **p < 0.01.
Exosomal miR-486 promoted angiogenesis of OGD/R-treated RBMECs through PTEN/Akt signaling pathway
The Akt signaling pathway has been linked to angiogenesis and can be negatively impacted by PTEN17. We further investigated whether miR-486 promotes angiogenesis via the PTEN/Akt signaling pathway. To determine the effectiveness of PTEN siRNAs, we carried out a Western blot assay and found that PTEN siRNA-2 had the most significant impact on PTEN expression, and selected it for further experimentation (Fig. 6C). Our subsequent results showed that PTEN siRNA significantly blocked the anti-angiogenic effects of miR-486 inhibitor-Exos (Fig. 7A–H). Furthermore, p-Akt/Akt expression was noticeably higher in the BMSC-Exos group than in the OGD/R group. However, when treated with miR-486 inhibitor-Exos, p-Akt/Akt expression decreased, but the inhibitory effect was markedly reversed by PTEN siRNA (Fig. 7I). In summary, our results demonstrate that BMSC-Exos promotes angiogenesis in OGD/R-treated RBMECs by regulating the miR-486/PTEN/Akt signaling pathway.
BMSC-Exos promotes RBMECs angiogenesis via miR-486/PTEN/Akt signaling pathway. (A–C) Representative images and quantification of scratch assay of RBMECs. Scale bar = 200 μm. (D, E) Representative images and quantification of transwell assay for RBMECs. Scale bar = 100 μm. (F–H) Representative images and quantification of tube formation ability of RBMECs. Scale bar = 100 μm. (I) Representative western blot images of p-Akt/Akt in the OGD/R- treated RBMECs and quantification of densitometries of Western blot band. The data are presented as the mean ± SD. n = 3. *p < 0.05, **p < 0.01.
Discussion
There is growing evidence that stem cell transplantation may be a promising approach for improving angiogenesis, and exosomes have been identified as the major paracrine factor of stem cell-induced angiogenesis31. Compared with stem cells, exosomes show the advantages of easy to cross the blood–brain barrier, low immunogenicity and high biocompatibility, and have a better application prospect in the treatment of central nervous system diseases32. Our present study found that BMSC-Exos improved neurological functional recovery and increased angiogenesis in cerebral ischemic rats. In vitro studies confirmed that BMSC-Exos promoted the proliferation, migration and tube formation of OGD/R-treated RBMECs. Further mechanistic studies revealed that BMSC-Exos promoted angiogenesis by regulating the PTEN/Akt pathway through transport of miR-486.
Exosomes contain a variety of bioactive molecules, including proteins, lipids, mRNAs, and miRs. Accumulating evidence indicates that exosomes exert therapeutic effects mainly through delivery of miRs. For instance, MSC-derived exosomes contributed to the angiogenesis of endothelial cells via miR-125a transfer33. Adipose stem cells-derived exosomes played an essential role in promoting angiogenesis of OGD-treated brain microvascular endothelial cells through miR-181b/TRPM7 axis34. Recently, we found that BMSC-Exos promoted angiogenesis in mice with ischemic stroke via miR-21-5p21. However, which miRs in BMSC-Exos are the crucial angiogenic factor inducing angiogenesis after cerebral ischemia needs to be further elucidated.
MiR-486 mediates the survival and angiogenic activity of MSCs under hypoxic conditions30. Several studies have reported that miR-486 is abundant in BMSC-Exos and can promote angiogenesis in various tissues, including the heart and skin24,25,35,36. In this study, we found that BMSC-Exos increased miR-486 expression and promoted angiogenesis in cerebral ischemic rats. BMSC-Exos treatment also promoted the proliferation, migration and lumen formation of OGD/R-treated RBMECs, and increased the level of miR-486. In vitro studies further demonstrated that miR-486 mimic-Exos enhanced the proliferation, migration, and tube formation of OGD/R-treated RBMECs, while miR-486 inhibitor-Exos had the opposite effects. These results suggest that BMSC-Exos promote angiogenesis after cerebral ischemia at least in part by transporting miR-486.
Previous studies have found that miR-486 protects against cardiomyocyte apoptosis by activating the PI3K/Akt pathway through targeting inhibition of PTEN37,38. Inhibition of PTEN has been shown to promote angiogenesis by increasing Akt phosphorylation39,40. Recent evidence indicated that miR-486 in small extracellular vesicles was involved in angiogenesis, as it appeared to stimulate this process by inhibiting PTEN expression and activating the Akt/mTOR/HIF-1α pathway41. We found that miR-486 overexpression decreased PTEN expression, while miR-486 knockdown increased PTEN expression in RBMECs. Furthermore, miR-486 mimic-Exos significantly increased miR-486 expression, while miR-486 inhibitor-Exos decreased miR-486 expression in OGD/R-treated RBMECs, so the association between miR-486 and PTEN expression may be RBMECs specific. Therefore, we evaluated the involvement of PTEN in miR-486-mediated angiogenesis of RBMECs. Our results demonstrated that the miR-486 inhibitor-Exos inhibited the proangiogenic activity and p-Akt expression of OGD/R-treated RBMECs, whereas PTEN siRNA reversed these effects. These data suggest that BMSC-Exos increases the pro-angiogenic activity of OGD/R-treated RBMECs by inhibition of PTEN expression and activation of Akt signaling through transferring miR-486.
However, there are still some deficiencies in this study. First, the effect and mechanism of exosomal miR-486 promoting angiogenesis needs to be further verified in animal models of cerebral ischemia. Second, multiple target genes of miR-486 have been reported to be involved in angiogenesis, such as MMP-19 and Sp524,25. Therefore, we cannot exclude the possibility that potential targets other than PTEN are involved in miR-486-induced angiogenesis.
Conclusions
In summary, our study indicates that BMSC-Exos promote angiogenesis after cerebral ischemia by regulating the PTEN/Akt pathway through the transfer of miR-486, which further reveals the mechanism of BMSC-Exos in the treatment of ischemic stroke.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Zhao, Y., Zhang, X., Chen, X. & Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 49, 1–9. https://doi.org/10.3892/ijmm.2021.5070 (2022).
Tang, H. et al. Endogenous neural stem cell-induced neurogenesis after ischemic stroke: Processes for brain repair and perspectives. Transl. Stroke Res. 14, 297–303. https://doi.org/10.1007/s12975-022-01078-5 (2023).
Mabotuwana, N. S. et al. Paracrine factors released by stem cells of mesenchymal origin and their effects in cardiovascular disease: A systematic review of pre-clinical studies. Stem Cell Rev. Rep. 18, 2606–2628. https://doi.org/10.1007/s12015-022-10429-6 (2022).
Zhou, L. et al. Potential mechanisms and therapeutic targets of mesenchymal stem cell transplantation for ischemic stroke. Stem Cell Res. Ther. 13, 195. https://doi.org/10.1186/s13287-022-02876-2 (2022).
Namiot, E. D., Niemi, J. V. L., Chubarev, V. N., Tarasov, V. V. & Schioth, H. B. Stem cells in clinical trials on neurological disorders: Trends in stem cells origins, indications, and status of the clinical trials. Int. J. Mol. Sci. 23, 11453. https://doi.org/10.3390/ijms231911453 (2022).
Asgari Taei, A., Khodabakhsh, P., Nasoohi, S., Farahmandfar, M. & Dargahi, L. Paracrine effects of mesenchymal stem cells in ischemic stroke: Opportunities and challenges. Mol. Neurobiol. 59, 6281–6306. https://doi.org/10.1007/s12035-022-02967-4 (2022).
Li, J. et al. Mesenchymal stem cell therapy for ischemic stroke: A look into treatment mechanism and therapeutic potential. J. Neurol. 268, 4095–4107. https://doi.org/10.1007/s00415-020-10138-5 (2021).
Suzuki, E., Fujita, D., Takahashi, M., Oba, S. & Nishimatsu, H. Stem cell-derived exosomes as a therapeutic tool for cardiovascular disease. World J. Stem Cells 8, 297–305. https://doi.org/10.4252/wjsc.v8.i9.297 (2016).
Lin, H. et al. Urinary exosomal miRNAs as biomarkers of bladder cancer and experimental verification of mechanism of miR-93-5p in bladder cancer. BMC Cancer 21, 1293. https://doi.org/10.1186/s12885-021-08926-x (2021).
Hade, M. D., Suire, C. N. & Suo, Z. Mesenchymal stem cell-derived exosomes: Applications in regenerative medicine. Cells 10, 1959. https://doi.org/10.3390/cells10081959 (2021).
Li, X., Bi, T. & Yang, S. Exosomal microRNA-150-5p from bone marrow mesenchymal stromal cells mitigates cerebral ischemia/reperfusion injury via targeting toll-like receptor 5. Bioengineered 13, 3030–3043. https://doi.org/10.1080/21655979.2021.2012402 (2022).
Han, Z. F. et al. Exosomal lncRNA KLF3-AS1 derived from bone marrow mesenchymal stem cells stimulates angiogenesis to promote diabetic cutaneous wound healing. Diabetes Res. Clin. Pract. 183, 109126. https://doi.org/10.1016/j.diabres.2021.109126 (2022).
Xiao, R. et al. BMSC-derived exosomal Egr2 ameliorates ischemic stroke by directly upregulating SIRT6 to suppress notch signaling. Mol. Neurobiol. 60, 1–17. https://doi.org/10.1007/s12035-022-03037-5 (2023).
Krupinski, J., Kaluza, J., Kumar, P., Kumar, S. & Wang, J. M. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 25, 1794–1798. https://doi.org/10.1161/01.str.25.9.1794 (1994).
Wei, L., Erinjeri, J. P., Rovainen, C. M. & Woolsey, T. A. Collateral growth and angiogenesis around cortical stroke. Stroke 32, 2179–2184. https://doi.org/10.1161/hs0901.094282 (2001).
Yang, H. et al. pH-sensitive, cerebral vasculature-targeting hydroxyethyl starch functionalized nanoparticles for improved angiogenesis and neurological function recovery in ischemic stroke. Adv. Healthc. Mater. 10, e2100028. https://doi.org/10.1002/adhm.202100028 (2021).
Deng, W. et al. MicroRNA-130a regulates neurological deficit and angiogenesis in rats with ischaemic stroke by targeting XIAP. J. Cell Mol. Med. 24, 10987–11000. https://doi.org/10.1111/jcmm.15732 (2020).
Wang, T. et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol. Direct. 18, 6. https://doi.org/10.1186/s13062-023-00361-1 (2023).
Zhang, Y. et al. MiR-17-92 cluster-enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J. Neurotrauma 38, 1535–1550. https://doi.org/10.1089/neu.2020.7575 (2021).
Pan, Q. et al. Exosomes derived from mesenchymal stem cells ameliorate hypoxia/reoxygenation-injured ECs via transferring MicroRNA-126. Stem Cells Int. 2019, 2831756. https://doi.org/10.1155/2019/2831756 (2019).
Hu, H. et al. Exosomes derived from bone marrow mesenchymal stem cells promote angiogenesis in ischemic stroke mice via upregulation of MiR-21–5p. Biomolecules 12, 883. https://doi.org/10.3390/biom12070883 (2022).
Baglio, S. R. et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 6, 127. https://doi.org/10.1186/s13287-015-0116-z (2015).
Douvris, A., Vinas, J. & Burns, K. D. miRNA-486-5p: Signaling targets and role in non-malignant disease. Cell Mol. Life Sci. 79, 376. https://doi.org/10.1007/s00018-022-04406-y (2022).
Li, Q. et al. Small extracellular vesicles containing miR-486–5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci. Transl. Med. 13, eabb0202. https://doi.org/10.1126/scitranslmed.abb0202 (2021).
Lu, Y. et al. Extracellular vesicle-enclosed miR-486-5p mediates wound healing with adipose-derived stem cells by promoting angiogenesis. J. Cell Mol. Med. 24, 9590–9604. https://doi.org/10.1111/jcmm.15387 (2020).
Li, L. et al. Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res. Ther. 8, 112. https://doi.org/10.1186/s13287-017-0565-7 (2017).
Li, L. et al. Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARgamma pathway after cerebral ischemia/reperfusion injury in rats. Int. Immunopharmacol. 92, 107335. https://doi.org/10.1016/j.intimp.2020.107335 (2021).
Chen, J. et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32, 1005–1011. https://doi.org/10.1161/01.str.32.4.1005 (2001).
Zhang, L. et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J. Neurosci. Methods 117, 207–214. https://doi.org/10.1016/s0165-0270(02)00114-0 (2002).
Shi, X. F. et al. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem. Biophys. Res. Commun. 470, 670–677. https://doi.org/10.1016/j.bbrc.2016.01.084 (2016).
Bian, D., Wu, Y., Song, G., Azizi, R. & Zamani, A. The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: A comprehensive review. Stem Cell Res. Ther. 13, 24. https://doi.org/10.1186/s13287-021-02697-9 (2022).
Xin, H. et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J. Cereb. Blood Flow Metab. 33, 1711–1715. https://doi.org/10.1038/jcbfm.2013.152 (2013).
Liang, X., Zhang, L., Wang, S., Han, Q. & Zhao, R. C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell. Sci. 129, 2182–2189. https://doi.org/10.1242/jcs.170373 (2016).
Yang, Y., Cai, Y., Zhang, Y., Liu, J. & Xu, Z. Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through MicroRNA-181b/TRPM7 axis. J. Mol. Neurosci. 65, 74–83. https://doi.org/10.1007/s12031-018-1071-9 (2018).
Cho, K. A., Park, M., Kim, Y. H., Woo, S. Y. & Ryu, K. H. RNA sequencing reveals a transcriptomic portrait of human mesenchymal stem cells from bone marrow, adipose tissue, and palatine tonsils. Sci. Rep. 7, 17114. https://doi.org/10.1038/s41598-017-16788-2 (2017).
Park, G. C. et al. Role of fibroblast growth factor-5 on the proliferation of human tonsil-derived mesenchymal stem cells. Stem Cells Dev. 25, 1149–1160. https://doi.org/10.1089/scd.2016.0061 (2016).
Sun, X. H., Wang, X., Zhang, Y. & Hui, J. Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb. Res. 177, 23–32. https://doi.org/10.1016/j.thromres.2019.02.002 (2019).
Zhu, H. H. et al. MicroRNA-486-5p targeting PTEN protects against coronary microembolization-induced cardiomyocyte apoptosis in rats by activating the PI3K/AKT pathway. Eur. J. Pharmacol. 855, 244–251. https://doi.org/10.1016/j.ejphar.2019.03.045 (2019).
Choorapoikayil, S., Weijts, B., Kers, R., de Bruin, A. & den Hertog, J. Loss of Pten promotes angiogenesis and enhanced vegfaa expression in zebrafish. Dis. Model Mech. 6, 1159–1166. https://doi.org/10.1242/dmm.012377 (2013).
Xue, L. et al. PTEN inhibition enhances angiogenesis in an in vitro model of ischemic injury by promoting Akt phosphorylation and subsequent hypoxia inducible factor-1alpha upregulation. Metab. Brain Dis. 33, 1679–1688. https://doi.org/10.1007/s11011-018-0276-5 (2018).
Shen, Z. et al. Small extracellular vesicles of hypoxic endothelial cells regulate the therapeutic potential of adipose-derived mesenchymal stem cells via miR-486-5p/PTEN in a limb ischemia model. J. Nanobiotechnol. 20, 422. https://doi.org/10.1186/s12951-022-01632-1 (2022).
Funding
This work was supported by the National Natural Science Foundation of China (No. 81274113, 81873028) and the Natural Science Foundation of Zhejiang Province (No. 2016C33185).
Author information
Authors and Affiliations
Contributions
H.B., S.M. and X.H. conceived and designed the study, performed the in vivo and in vitro experiments, analyzed the data and wrote the manuscript. L.L., H.T. and J.Z. performed in vitro experiments and analyzed the data. L.X., Y.F. and Y.Z. analyzed and interpreted the data. L.C. contributed to the study design, data analysis and interpretation, and manuscript writing and revision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bao, H., Mao, S., Hu, X. et al. Exosomal miR-486 derived from bone marrow mesenchymal stem cells promotes angiogenesis following cerebral ischemic injury by regulating the PTEN/Akt pathway. Sci Rep 14, 18086 (2024). https://doi.org/10.1038/s41598-024-69172-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69172-2
- Springer Nature Limited