Abstract
Relative fat mass (RFM) is a novel indicator for measuring body fat. This cross-section study aims to explore the association between RFM and periodontitis and to investigate possible effect modifiers in U.S. adults based on the National Health and Nutrition Examination Survey 2009–2014. The category of periodontitis was defined by the CDC/AAP. Mean clinical attachment loss and mean pocket probing depth (PPD) were calculated. The RFM formula is: 64 − (20 × height/WC) + (12 × sex), with sex coded as 1 for female and 0 for male. Natural cubic spline and weighted multivariable regression analyses were conducted to investigate the relationship between RFM and periodontal status. Subgroup and interaction analyses were also employed to assess the moderating roles of age, gender, and race. A total of 10,307 participants were included in our study. Compared to the lowest quartiles, individuals in the highest quartiles of RFM levels were more likely to have moderate/severe periodontitis (ORQ4vs1 = 1.64, 95% CI 1.30–2.06) and had a higher mean PPD (βQ4vs1 = 0.15, 95% CI 0.09–0.22). This association was particularly stronger in populations under the age of 60, with significant interactions. Taken together, RFM is positively associated with periodontitis, particularly in those under 60 years old.
Similar content being viewed by others
Introduction
Periodontitis is a widespread health concern, with over 50% of adults globally afflicted by the condition. It is characterized by the breakdown of the soft and hard tissues surrounding the teeth, primarily caused by bacteria present in dental plaque. Additionally, the host immune response plays a crucial role in the onset and progression of periodontitis1,2. Notably, obesity can affect the host-microbial homeostasis, increasing the risk of developing periodontitis3,4.
Obesity is a metabolic condition resulting from an energy imbalance (consumption < intake), leading to an increase in adipose tissue deposits. While the most common method uses body mass index (BMI) as an indicator of obesity, BMI does not account for frame size or differentiate between fat mass, bone mass, and muscle mass5,6. To provide more accurate anthropometric measures of adiposity, various indices such as body shape index, body shape index, and weight-adjusted waist index have been developed. Recently, a simple new algorithm called relative fat mass (RFM) has been introduced to estimate whole-body fat percentage in adults7. RFM has been shown to be the strongest predictor of heart failure risk in the general population8. Additionally, higher RFM is associated with hypertension9, depression10, and type 2 diabetes11.
RFM does not have the disadvantages of previously used measures of overweight and obesity. Thus, in this cross-sectional study, we aimed to explore the association between RFM and periodontitis and to investigate possible effect modifiers in U.S. adults based on the National Health and Nutrition Examination Survey (NHANES) 2009–2014.
Methods
Study population
The data utilized in this cross-sectional study were obtained from the NHANES 2009–2014 survey, conducted by the National Center for Health Statistics (NCHS) to ensure representation of the national population. NHANES is a stratified, multistage, clustered probability sampling study that gathered data through interviews as well as physical and laboratory examinations12,13. For this study, we excluded individuals who did not undergo a complete full-mouth periodontal examination (FMPE), those for whom data on assessing RFM was unavailable, and individuals under the age of 30. We reported this study following the STROBE criteria for observational studies in epidemiology14.
Exposure variable
Height and waist circumference (WC) were assessed by a health professional at the Mobile Examination Center (MEC) to determine the Ratio of Fat Mass (RFM), considering the participant's sex. Participants stood barefoot against a special height measuring device at the MEC, ensuring their heads were level with their backs to the board. WC was measured at the line above the iliac crest in the mid-axillary line at the end of normal breathing, with measurements precise to within 0.1 cm10. The units for height and WC were both in centimeters. The RFM formula is: 64 − (20 × height/WC) + (12 × sex), with sex coded as 1 for female and 0 for male7.
Outcome variable
The study's outcome was defined as moderate to severe periodontitis. The periodontal assessment involved measuring clinical attachment loss (CAL) and probing pocket depth (PPD) at six predetermined sites on each tooth, following the FMPE protocol and excluding the third molars. CAL refers to the distance between the cemento-enamel junction and the base of the sulcus, whereas PPD is the measurement from the free gingival margin to the base of the sulcus. The classification of periodontitis was based on the definitions provided by CDC/AAP15. No/mild periodontitis was classified as the absence of any indication of moderate/severe periodontitis; moderate periodontitis was defined as having ≥ 2 interproximal sites with a PPD of ≥ 5 mm that were not on the same tooth, or ≥ 2 interproximal sites with a AL of ≥ 4 mm that were not on the same tooth; severe periodontitis was characterized by having ≥ 2 interproximal sites with a AL of ≥ 6 mm that were not on the same tooth, along with at least one interproximal site with a PPD of ≥ 5 mm. Furthermore, we calculated the average values for CAL and PPD to provide a comprehensive understanding of periodontal health.
Covariates
Covariates available in the NHANES database were collected based on previous studies16,17, including age (≤ 60 or > 60 years), sex (male or female), race (non-Hispanic White, non-Hispanic Black, Mexican American or others), marital status (married/living as married, never married or separated/divorced/widowed), smoking status (non-smokers, former-smokers or smokers), recreational activity (vigorous activity, moderate activity or other), work activity (vigorous activity, moderate activity or other), frequency of weekly use of floss/device, diabetes mellitus and hypertension. The data on moderate/vigorous recreational activity and moderate/vigorous work activity were collected based on questionnaire. The diagnosis of diabetes mellitus was established using criteria such as self-reported physician diagnosis, a fasting blood glucose level ≥ 7.0 mmol/L, a glycosylated haemoglobin level (HbA1c) ≥ 6.5%, or current use of hypoglycaemic drugs. Similarly, the diagnosis of hypertension was determined based on self-reported physician diagnosis, a diastolic blood pressure measurement of ≥ 80 mmHg, a systolic blood pressure reading of ≥ 130 mmHg, or current use of antihypertensive medications.
Statistical analysis
Weights were determined in our study following the guidelines outlined in the NHANES analysis guide. The calculation of the new 6-year weights was derived from 1/3 of the 2-year MEC weights. Continuous variables were presented using mean and standard error (SE), while categorical variables were described using proportions. To compare baseline characteristics across different RFM levels, analysis of variance (ANOVA) was conducted for continuous variables and the chi-square test for categorical variables.
To avoid arbitrary categorization, restricted cubic splines with four knots was utilized to examine the potential nonlinear relationship of RFM and periodontitis. Two weighted multivariable models were applied to investigate the association between RFM and periodontal health, taking into account possible confounding factors. Model I was adjusted for age, sex, and race, while model II included additional adjustments for marital status, smoking status, recreational activity, work activity, frequency of weekly use of floss/device, diabetes, and hypertension. The weighted odds ratio (OR) and 95% confidence interval (CI) were obtained based on weighted logistic regression analysis, while the weighted β and 95% CI were obtained based on weighted linear regression analysis. Furthermore, stratified analyses and interaction analyses were conducted to assess whether the association between RFM and periodontitis differed by age, sex, and race. In the racial subgroup analysis, we divided the participants into two groups: Non-Hispanic White and Others (Non-Hispanic Black, Mexican American, or others). Additionally, we observed a J-shaped association between the systemic immune-inflammation index (SII) and periodontitis in our previous study, with an inflection point at log2(SII) of 8.6618. Considering the role of obesity-induced low-grade inflammation, we further examined the differences in RFM among different SII and age groups.
We also conducted a sensitivity analysis based on another periodontal classification proposed by the 2017 World Workshop on Periodontal and Peri-Implant Diseases and Conditions19. The severity of periodontitis was classified into four stages based on the maximum AL: 1–2 mm was classified as Stage I, 3–4 mm as Stage II, and equal to or greater than 5 mm as Stage III/IV. Additionally, management complexity was also taken into account. Patients diagnosed with Stage II periodontitis were reclassified as Stage III if their maximum PPD measurement was ≥ 6 mm. Furthermore, patients diagnosed with Stage III periodontitis were reclassified as Stage IV if they have less than 20 teeth remaining. Stage III/IV periodontitis were set as the outcome; others were set as the reference. A complete case analysis approach was employed in our study owing to the negligible amount of missing data present. All statistical analyses were carried out using R software (version 4.1.2). A significance level of 0.05 was used to determine statistical significance.
Ethics approval and consent to participate
NHANES protocols were approved by the Research Ethics Review Board of the National Center for Health Statistics, U.S. Centers for Disease Control and Prevention (NCHS IRB/ERB Protocol Number: 2009–2010, Protocol #2005–06; 2011–2014, Protocol #2011–17), and data collection was carried out in accordance with the aforementioned protocol. Written informed consent was obtained from all participants.
Results
Baseline characteristics of study samples
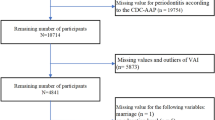
As shown in Fig. 1, a total of 10,307 individuals were included in this study, representing an estimated population of approximately 139.5 million non-institutionalized U.S. residents. Table 1 presents the baseline characteristics of the individuals in the study. The average age of participants was 50.68 years, with 50.85% being female. Significant differences were noted in various factors among the different RFM levels, including average age, sex, race, marital status, smoking habit, work activity, recreational activity, frequency of weekly use of floss/device, prevalence of diabetes, hypertension, periodontitis, mean CAL, and mean PPD. The prevalence of moderate/severe periodontitis across the RFM levels was reported as follows: 39.78%, 40.91%, 33.67%, and 35.52%, respectively.
Association between RFM and periodontitis
As depicted in Fig. 2, RFM exhibits a non-linear positive relationship with moderate/severe periodontitis (Pnon-linearity = 0.02). The risk of periodontitis showed a slight declining trend before RFM reached around 30, and then increased rapidly thereafter until RFM was around 43. Consequently, RFM was categorized into quartiles for further analyses. Initially, an unadjusted model indicated a negative association between RFM and moderate/severe periodontitis (ORQ3vs1 = 0.77, 95% CI 0.65–0.90; ORQ4vs1 = 0.83, 95% CI 0.72–0.97). However, in the fully adjusted model, a positive association between RFM and periodontitis was observed (ORQ3vs1 = 1.50, 95% CI 1.24–1.83; ORQ4vs1 = 1.64, 95% CI 1.30–2.06) (Table 2). Similarity, RFM demonstrated a positive association with mean PPD (βQ3vs1 = 0.11, 95% CI 0.05–0.17; βQ4vs1 = 0.15, 95% CI 0.09–0.22). There was a positive association between RFM and mean CAL after adjusting for age, sex, and race (βQ3vs1 = 0.14, 95% CI 0.02–0.27; βQ4vs1 = 0.22, 95% CI 0.10–0.33). However, no significant association of RFM with mean CAL was found in the fully adjusted model (Table 3).
Subgroup analyses
Age significantly modified the relationship between RFM and periodontitis, revealing distinctive associations across different age groups. Specifically, individuals younger than 60 years old, RFM showed a significant relationship with periodontitis (ORQ3vs1 = 1.67, 95% CI 1.27–2.19; ORQ4vs1 = 2.21, 95% CI 1.64–2.96). In contrast, a trend towards an inverse relationship between RFM and periodontitis was observed among older adults (ORQ4vs1 = 0.68, 95% CI 0.41–1.15) (Table 4 and Fig. S1). In addition, RFM showed a significant association with periodontitis in males (ORQ3vs1 = 1.54, 95% CI 1.13–2.09), as well as in Whites (ORQ4vs1 = 1.87, 95% CI 1.34–2.61) and individuals of other races (ORQ4vs1 = 1.54, 95% CI 1.13–2.11). Considering the role of obesity-induced low-grade inflammation, we further explored the difference in RFM between SII and age groups. The results revealed that individuals under 60 years old with a more active immune response exhibited higher RFM levels. Conversely, in older adults, there was no apparent association between host immune response and RFM (Fig. S2).
Sensitivity analyses
A sensitivity analysis was employed to assess the relationship between RFM and Stage III/IV periodontitis. The results also revealed a similar trend concerning this relationship (Fig. S3 and Table S1). The highest quartiles of RFM decreased the risk of Stage III/IV periodontitis compared to quartiles 1 in the crude model (ORQ4vs1 = 0.73, 95% CI 0.62–0.87). However, in the fully adjusted model, the highest quartiles of RFM increased the risk of Stage III/IV periodontitis compared to quartiles1 (ORQ4vs1 = 1.36, 95% CI 1.01–1.83).
Discussion
In this cross-sectional study, we identified a non-linear positive relationship between RFM and periodontitis. The association was more pronounced when RFM was approximately 30–43. The stronger associations of RFM with periodontitis were observed in individuals younger than 60 years old. RFM may be suggested as a convenient and suitable measure of obesity for investigating the relationship between obesity and periodontitis. However, additional longitudinal trials are necessary to provide further evidence and support for our findings.
Increasing evidence indicates that obesity is a risk factor for periodontitis. Adipose tissue serves as a significant source of various pro-inflammatory mediators, while the reduced production of anti-inflammatory adipokines results in a systemic imbalance between pro- and anti-inflammatory adipokines, leading to a sustained low-grade inflammatory state20,21. In our previous study, we observed a J-shaped association between the SII and periodontitis18, suggesting a potential explanation for the inverse association trend between RFM and periodontitis when RFM is below 30. Furthermore, obesity leads to an increase in reactive oxygen species (ROS) and a decrease in antioxidant capacity22. Elevated levels of ROS can cause damage to DNA, lipids, and proteins in host cells, leading to cytotoxicity and accelerating the advancement of periodontitis23.
BMI is currently the most commonly utilized tool for diagnosing obesity, reflecting overall obesity. Recent studies have found that central obesity is more strongly associated with disease than overall obesity, including periodontitis24. As a result, several indices representing central obesity have been developed. Among these indices, RFM is easy to calculate, derived from waist circumference and height, and is sex-specific. It also performs well in estimating the proportion of total body fat, as validated by dual-energy X-ray absorptiometry (DXA)7,25. In line with BMI and WC26,27,28, we also found a positive association between RFM and periodontitis.
In subgroup analysis, a modifying effect of age on the relationship between RFM and periodontitis was observed. Additionally, a L-shaped association between RFM and periodontitis was noted in individuals under 60 years old, while older adults showed a negative association trend. Similar trends were also found between BMI and periodontitis28,29. Individuals under 60 years old with a more active immune response had higher RFM, while the host immune response was not associated with RFM in older adults, which may be a contributing factor to this phenotype. Furthermore, the dilution of the effect of obesity was observed in the older age groups. It is possible that the relationship between obesity and the periodontal condition of older participants was overshadowed by stronger risk factors, such as age28. However, it is still necessary to explore the molecular mechanism underlying the potential relationship between obesity and periodontitis in older adults.
This study presents several notable strengths. Firstly, the analysis was carried out on a substantial sample size, enhancing the reliability of the findings. Moreover, RFM is simpler to evaluate compared to DXA and does not involve radiation, making it more practical for use in clinical settings. Notably, the study is pioneering in uncovering a positive correlation between RFM and periodontal disease. Nevertheless, it is important to acknowledge various limitations that require consideration. Primarily, the cross-sectional design of the study precludes definitively establishing a causal relationship between RFM and periodontal health. Additionally, the study's data are specific to the U.S. population and may not be generalizable to other countries. In analytic epidemiology, achieving high internal validity is crucial. Therefore, future studies should prioritize this aspect over national representativeness of the data (external validity). Lastly, the presence of unmeasured variables impeded the full adjustment for all potential confounding factors. To address these limitations, future research endeavors should involve multi-center prospective trials to verify any potential causal associations.
Conclusions
RFM is positively associated with periodontitis in U.S. adults, particularly in those under 60 years old.
Data availability
The NHANES data of our study are openly available at https://www.cdc.gov/nchs/nhanes/default.aspx.
References
Kinane, D. F., Stathopoulou, P. G. & Papapanou, P. N. Periodontal diseases. Nat. Rev. Dis. Primers 3, 17038. https://doi.org/10.1038/nrdp.2017.38 (2017).
Tonetti, M. S. et al. Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 42(Suppl 16), S5-11. https://doi.org/10.1111/jcpe.12368 (2015).
Suvan, J. E. et al. Association between overweight/obesity and increased risk of periodontitis. J. Clin. Periodontol. 42, 733–739. https://doi.org/10.1111/jcpe.12421 (2015).
Gimeno, R. E. & Klaman, L. D. Adipose tissue as an active endocrine organ: recent advances. Curr. Opin. Pharmacol. 5, 122–128. https://doi.org/10.1016/j.coph.2005.01.006 (2005).
Rothman, K. J. BMI-related errors in the measurement of obesity. Int. J. Obes. (Lond.) 32(Suppl 3), S56-59. https://doi.org/10.1038/ijo.2008.87 (2008).
Humphreys, S. The unethical use of BMI in contemporary general practice. Br. J. Gen. Pract. 60, 696–697. https://doi.org/10.3399/bjgp10X515548 (2010).
Woolcott, O. O. & Bergman, R. N. Relative fat mass (RFM) as a new estimator of whole-body fat percentage—A cross-sectional study in American adult individuals. Sci. Rep. 8, 10980. https://doi.org/10.1038/s41598-018-29362-1 (2018).
Suthahar, N. et al. Relative fat mass, a new index of adiposity, is strongly associated with incident heart failure: data from PREVEND. Sci. Rep. 12, 147. https://doi.org/10.1038/s41598-021-02409-6 (2022).
Yu, P., Huang, T., Hu, S. & Yu, X. Predictive value of relative fat mass algorithm for incident hypertension: A 6-year prospective study in Chinese population. BMJ Open 10, e038420. https://doi.org/10.1136/bmjopen-2020-038420 (2020).
Zhu, X. et al. The relationship between depression and relative fat mass (RFM): A population-based study. J. Affect. Disord. https://doi.org/10.1016/j.jad.2024.04.031 (2024).
Suthahar, N. et al. Associations of relative fat mass, a new index of adiposity, with type-2 diabetes in the general population. Eur. J. Intern. Med. 109, 73–78. https://doi.org/10.1016/j.ejim.2022.12.024 (2023).
Curtin, L. R. et al. National health and nutrition examination survey: Sample design, 2007–2010. Vital Health Stat. 2, 1–23 (2013).
Johnson, C. L., Dohrmann, S. M., Burt, V. L. & Mohadjer, L. K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. 2, 1–33 (2014).
Ghaferi, A. A., Schwartz, T. A. & Pawlik, T. M. STROBE reporting guidelines for observational studies. JAMA Surg. 156, 577–578. https://doi.org/10.1001/jamasurg.2021.0528 (2021).
Eke, P. I., Page, R. C., Wei, L., Thornton-Evans, G. & Genco, R. J. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 83, 1449–1454. https://doi.org/10.1902/jop.2012.110664 (2012).
Cao, R., Li, A., Geng, F. & Pan, Y. Associations of dietary antioxidant intake with periodontal health among US adults: An exploratory mediation analysis via mitochondrial function. J. Clin. Periodontol. https://doi.org/10.1111/jcpe.13960 (2024).
Chen, H., Zhang, X., Luo, J., Dong, X. & Jiang, X. The association between periodontitis and lung function: Results from the National Health and Nutrition Examination Survey 2009 to 2012. J. Periodontol. 93, 901–910. https://doi.org/10.1002/jper.21-0399 (2022).
Cao, R., Li, C., Geng, F. & Pan, Y. J-shaped association between systemic immune-inflammation index and periodontitis: Results from NHANES 2009–2014. J. Periodontol. https://doi.org/10.1002/jper.23-0260 (2023).
Papapanou, P. N. et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 45(Suppl 20), S162-s170. https://doi.org/10.1111/jcpe.12946 (2018).
Adamczak, M. & Wiecek, A. The adipose tissue as an endocrine organ. Semin. Nephrol. 33, 2–13. https://doi.org/10.1016/j.semnephrol.2012.12.008 (2013).
Krysiak, R., Handzlik-Orlik, G. & Okopien, B. The role of adipokines in connective tissue diseases. Eur. J. Nutr. 51, 513–528. https://doi.org/10.1007/s00394-012-0370-0 (2012).
Tomofuji, T. et al. Effects of obesity on gingival oxidative stress in a rat model. J. Periodontol. 80, 1324–1329. https://doi.org/10.1902/jop.2009.080621 (2009).
Kanzaki, H. et al. Pathways that regulate ROS Scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front. Physiol. 8, 351. https://doi.org/10.3389/fphys.2017.00351 (2017).
Han, D. H., Lim, S. Y., Sun, B. C., Paek, D. M. & Kim, H. D. Visceral fat area-defined obesity and periodontitis among Koreans. J. Clin. Periodontol. 37, 172–179. https://doi.org/10.1111/j.1600-051X.2009.01515.x (2010).
Guzmán-León, A. E. et al. External validation of the relative fat mass (RFM) index in adults from north-west Mexico using different reference methods. PLoS One 14, e0226767. https://doi.org/10.1371/journal.pone.0226767 (2019).
Khader, Y. S., Bawadi, H. A., Haroun, T. F., Alomari, M. & Tayyem, R. F. The association between periodontal disease and obesity among adults in Jordan. J. Clin. Periodontol. 36, 18–24. https://doi.org/10.1111/j.1600-051X.2008.01345.x (2009).
Suvan, J., D’Aiuto, F., Moles, D. R., Petrie, A. & Donos, N. Association between overweight/obesity and periodontitis in adults. A systematic review. Obes. Rev. 12, e381-404. https://doi.org/10.1111/j.1467-789X.2010.00808.x (2011).
Liu, L. et al. Association between obesity and periodontitis in US adults: NHANES 2011–2014. Obes. Facts 17, 47–58. https://doi.org/10.1159/000534751 (2024).
Carneiro, D. O. et al. Obesity in young women is positively associated with periodontitis. Clin. Oral Investig. 26, 6139–6149. https://doi.org/10.1007/s00784-022-04563-1 (2022).
Acknowledgements
This study was supported by the Natural Science Foundation of Hunan Province (2021JJ40390).
Author information
Authors and Affiliations
Contributions
Study conception and design: R.C. and S.Z. Data collection, analysis, and figure preparation: L.Z. Manuscript writing: L.Z., R.C., and S.Z. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhao, L., Cao, R. & Zhang, S. Association between relative fat mass and periodontitis: results from NHANES 2009–2014. Sci Rep 14, 18251 (2024). https://doi.org/10.1038/s41598-024-69048-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69048-5
- Springer Nature Limited