Abstract
This retrospective study analyzed a large population of gastric cancer (GC) patients treated between 2010 and 2015 to investigate the clinical features and predictive risk factors for developing secondary primary malignancies (SPMs). The cumulative incidence of SPM was assessed using Kaplan–Meier analysis. Competing risk analyses adjusted for mortality were conducted using stratified Cox proportional hazard regression models and multivariate analyses to identify independent predictors of SPM. A total of 3289 out of 167,747 GC patients were included in the analytic cohort, with 155 patients diagnosed with SPM. Patients whose histologic type other than adenocarcinomas (AC) and signet ring cell carcinoma (SRCC) emerged as an independent risk factor for developing SPM (hazard ratio [HR] 2.262, 95% confidence interval [CI] 1.146–4.465, P = 0.019) in multivariate Cox regression analysis. The surgical method, including biopsy/local excision (HR 2.3, [CI] 1.291–4.095, P = 0.005) and subtotal/total resection ([HR] 1.947, [CI] 1.028–3.687, P = 0.041), chemotherapy ([HR] 1.527, [CI] 1.006–2.316, P = 0.047), and histologic type ([HR] 2.318, [CI] 1.193–4.504, P = 0.013)), were identified as independent risk factors in the competitive risk model. Subgroup analyses, stratified by chemotherapy, revealed an increased risk of SPM among older patients. Furthermore, a nomogram was developed and internally validated to predict the cumulative incidence of SPM in GC patients (C-index = 0.73 for 72 months). These findings suggested that in specific histologic types of GC, the lymph node infiltration region missed after local surgical resection, and concomitant chemotherapy would have an increased risk of SPM for cancer survivors.
Similar content being viewed by others
Introduction
In recent decades, there has been brilliant development in gastric cancer diagnosis and treatment, as evidenced by the growing proportion of early-stage gastric cancer and improved survival rates1. Randomized controlled trials and observational research have proven that endoscopic screening enables the detection of more early-stage patients, offering a substantial potential for reducing the burden of gastric cancer2,3. The percentage of T1 cancers has constantly expanded from 30.4% in 1995 to 61% in 2014, according to a nationwide survey in South Korea4. Current guidelines for gastric cancer therapy have shown that combined modality therapy (CMT), such as laparoscopic gastrectomy, can drastically amplify survival in gastric cancer patients, and postoperative chemoradiotherapy (CRT) or perioperative chemotherapy (CT) are the desired procedures for treating localized gastric cancer5,6. Nevertheless, since most gastric adenocarcinoma has the characteristics of chromosomal instability and high level of copy number changes, which makes it immune exclusion, the treatments for these immunologically cold gastric cancers include ICI-chemotherapy, such as nivolumab and oxaliplatin-based chemotherapy7, and ICI-targeted therapy, such as ICIs combined HER2-targed treatments, and even ICI-targeted-chemotherapy combinations8. This advancement is anticipated to further enhance the survival rate of patients. However, there may be a stronger focus on exploring the therapeutic benefits of agents for survival rather than prioritizing the research on the new and inevitable adverse events10, as well as the currently unknown long-term effects. Patients with prolonged survival times and a higher proportion of survivors face an increased risk of developing secondary malignancies (SPM)11. The presence of SPM poses a challenge as patients with SPMs generally present lower survival rates than those without it12. To date, there is limited information available regarding the role of pathology and treatment options in SPM models, and additional efforts are required to acquire a more comprehensive understanding of the promising yet under-researched subject of GC management.
SPM are late complications arising after exposure to genotoxins13. It is no longer feasible to expect which sufferers will develop SPM, nor are there recognized mechanisms explaining the pathogenesis of different SPM histologies. The incidence of SPM is increasing, mostly since the growing population of younger cancer survivors in recent decades. For SPM in gastric carcinoma, 235 secondary malignancies (2.32%) were reported amongst 10,138 patients who underwent gastrectomy14. The ratio is elevated to that of 2018 SPMs among 33,705 (5.99%) patients in the study by Li et al.15. The chance of SPM is influenced by factors such as the patient’s age, cancer stage at diagnosis, and cure modality16. However, for a significant number of patients with an early remedy of stomach cancer, the occurrence of secondary malignant tumors has not been well-documented. Knowledge of which elements are the hazard of growing therapy-induced secondary malignancies could guide noninvasive monitoring for secondary malignancy after early-stage GC therapy.
In this study, we retrospectively analyzed and sorted out the clinicopathological points and unbiased prognostic factors of patients with SPMs. Based on the recognized prognostic elements by Cox proportional risk model and competitive-risk model, the nomogram had been built to predict the incidence of SPM. Overall, our findings may single out the comprehensive scientific elements of patients with SPM and offer more detailed insight into the risk factors for SPM.
Methods
Data sources
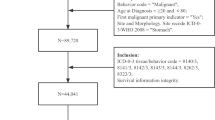
Data analyzed in this study have been received from the Surveillance, Epidemiology, and End Results (SEER) application of the United States National Cancer Institute (https://seer.cancer.gov/), which provides information on cancer incidence, prevalence, mortality, and evidence-based scientific findings across various US states covering a period of decades17. Patient demographic and clinical data released in 2010–2015 were downloaded using the SEER*Stat software program (version 8.3.9; National Cancer Institute, Bethesda, MD, USA). A total of 159,897 patients with a pathologically confirmed diagnosis of gastric cancer were initially included for analysis. Primary gastric cancer was defined according to the criteria established by the International Agency for Research on Cancer18. According to the SEER database coding manual, all patients with Histologic Type, coded by the International Classification of Diseases for Oncology (ICD-O-3) of 8140, 8211, 8260, 8480 and 8490 gastric adenocarcinoma, and TNM classification confined to T1N0M0, were included. Patients with unknown information on survival time, surgery, radiation, and region nodes were excluded. In addition, patients whose secondary tumor interval equal to zero were also excluded. Finally, 3289 patients with secondary malignant tumors were enrolled in the primary cohort (Supplementary Fig. S1).
Variable declaration
This study compared different clinicopathologic factors between the second malignancies (SPM) and non-SPM groups using Pearson’s chi-squared test for categorical variables. Secondary malignancy was defined as the subsequent first primary cancer, occurring at least two months after the first cancer diagnosis19. The SEER database manual provides detailed definitions of all variables used in this study. Continuous variables such as age, period of case inclusion, and involvement of regional lymph nodes were converted into categorical variables. The main endpoint for evaluation was the occurrence of SPM. The Kaplan–Meier curve was used to show differences in the incidence of secondary malignancy between different groups. Hazard ratios have been estimated by fitting a Cox proportional hazards model (proportional hazards have been examined and showed no significant, see the Supplementary Table S1). Univariate and multivariate Cox regression analyses were used to discover the prognostic value of these factors.
The fine-gray proportional hazards modeling
We used a competing-risk model to extend the proportional hazards model and estimate the risk of SPM according to the Fine-Gray model. Briefly, we regarded SPM as the result of interest, whereas death events were considered competing for risk events, and an affected patient alive was considered a censored event. The cumulative hazard of the outcome variables was calculated using the cumulative hazard function, and the cumulative incidence function (CIF) for the covariates was plotted20. Exploratory one-way subgroup analysis was performed using the Gray model to analyze the effect of chemotherapy on patient prognosis in different subgroups of each covariate, and the likelihood ratio test (LRT) was used to analyze the interaction between chemotherapy and other covariates.
Competing-risk nomogram construction and evaluation
Eligible patients were randomized into training and testing sets at a ratio of 1:1. A clinical predictive model based on a COX regression model was constructed in the training set. The Stepwise method was applied to filter variables by “regplot” R package. The independent predictive factors have been used to construct the nomogram for predicting SPM by using the “rms” package in R.
The nomogram was evaluated in the training set. Model performance for predicting the SPM consequences was evaluated by calculating the Harrell concordance index (C-index) and the area under the curve (AUC) of the receiver operating characteristic curve (ROC)21. Calibration analysis was used to visualize the accurate evaluation of the model22, while the brier score was used to evaluate the discrimination and calibration degree. Additionally, the decision curve analysis (DCA) was used to consider the clinical benefit23.
Statistical analysis
The data were analyzed and visualized using R software version 4.1.2. The following R packages were also utilized: survminer, mstate, riskRegression, rms, pec, cmprsk, and foreign. All tests were 2-sided with a P value less than 0.05 considered statistically significant.
Ethics approval
All authors have utilized and bought permission to use the database from SEER’s authentic website. The ethics committee requires no assessment and content material to use the SEER database.
Results
Demographic and clinical characteristics of patients
Clinical and demographic details of the patients are summarized in Table 1. In the GC cohort, a total of 3134 patients were stratified into the NSPM group, and 155 into the SPM group. Among the variables, we found that diagnosis during different periods (P = 0.005), different AJCC T stage (P = 0.012), histologic type (P = 0.037) of tumor and surgery methods (P = 0.003) were significantly different between the two groups based on the Fisher exact test (P < 0.05). The general variables of the patients in the NSPM and SPM groups were further analyzed by competitive risk model (Table 2), revealing significant differences in almost every variable (P < 0.01) except for gender (P = 0.345). Among the cases of SPM, stomach cancer (15.48%), lung and bronchus cancer (10.97%), and prostate cancer (9.68%) exhibited the highest incidence rates (Fig. 1A). The frequency of secondary tumors in each cancer species is shown in Fig. 1B, with stomach cancer (24 cases), lung and bronchus cancer, and prostate cancer being the three cancers with the highest frequency. The Kaplan–Meier curve for the SPM was significantly different in histologic types (P < 0.05), regional nodes (P = 0.013), marital status (P = 0.034), and tumor primary site (P = 0.013) (Supplementary Fig. S2).
Univariable and multivariable cox regression analyses in patients with SPM
SPM were analyzed by the univariate and multivariate Cox proportional hazards models (Fig. 2). In the Univariate Cox regression analysis, two general variables, namely fewer regional nodes and histologic type other than SRCC, were found to be individually associated with improved SPM. Subsequently, the regional nodes and histologic type of SPM were further examined in the multivariate Cox regression analysis. Patients with a histologic type other than SRCC of gastric cancer were found to have an increased risk of SPM compared to those with SRCC (HR 2.262, 95% CI 1.146–4.465, P = 0.019), after considering regional nodes involvement.
Sensitivity analyses and cumulative incidence of a SPM
The Forest plot (Fig. 3A) confirmed the subdistribution hazard ratios estimated from the Fine and Gray competing risk regression model. In the univariate analysis of SPM, T1NOS, fewer regional nodes, surgical treatment, and histologic type other than AC and SRCC were individually associated with an increased risk of SPM. In the multivariate analysis, after multivariate adjustment, both other histologic type (HR 2.318, 95% CI 1.193–4.504, P = 0.013) and surgery methods such as biopsy/local excision (HR 2.3, 95% CI 1.291–4.095, P = 0.005) and subtotal and total resection (HR 1.947, 95% CI 1.028–3.687, P = 0.041) were identified as independent risk factors for SPM. It is noteworthy that patients who received chemotherapy had an estimated 50% amplify risk of SPM (HR 1.527; 95% CI 1.006–2.316; P = 0.047) (Fig. 3B). CIF curves for all variables are shown in Supplementary Fig. S3. The cumulative incidences of SPM and death grouped by different variables are presented (Fig. 4). The results showed that the CIF curve of the covariates, and by the univariate Gray’s test, cumulative incidences of SPM were significantly different in patients with different age, race, marital status, grade, regional node, AJCC stage, chemotherapy, radiotherapy and primary site significantly correlated with the cumulative risk of secondary malignancy (P < 0.001), while surgery method, AJCC T1 substage and histologic type were associated with both the cumulative risk of SPM and death ascompetitive events(P < 0.05). In COX regression analysis, the AUC values suggested that the diagnosis period (0.73), regional nodes (0.63) and surgical methods (0.6) could predict SPM well at 72 months (Supplementary Table S2). However, the AUC values for the diagnosis period (0.26) and regional nodes (0.37) predict SPM poorly in the competitive risk model (Supplementary Table S3).
Univariate subgroup analysis
In the univariate subgroup analysis (Fig. 4), chemotherapy status showed a significant association with SPM in the age 18–65 years subgroup, with a sHR (95% CI) of4.21 (3.039, 5.833), P < 0.001. In the age 66–75 years subgroup, chemotherapy was also significantly associated with secondary malignancy, with a sHR (95% CI) of 2.508 (1.848, 3.405), P < 0.001; the P value of the interaction test was 0.018. In the 76–100 year subgroup, the sHR (95% CI) for chemotherapy was1.538 (1.24, 1.909), with a P < 0.001; the P value of the interaction test was less than 0.05. This suggests that there is a significant interaction between chemotherapy and age. In addition, being widowed (sHR 1.401, 95% CI 1.047–1.875, P = 0.003), having an AJCC IB stage (sHR 1.312, 95% CI 0.94–1.832, P = 0.01), and undergoing surgical biopsy/local excision treatment (sHR 6.222, 95% CI 2.455–15.766, P = 0.001) were positively correlated with SPMs. On the other hand, radiotherapy (sHR 0.614, 95% CI 0.453–0.834, P < 0.001) was negatively correlated with SPMs.
Competing-risk nomogram construction and validation
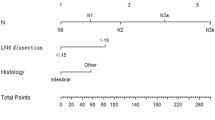
A predictive nomogram based on the Cox regression model was set up to predict the probabilities of SPM at 24, 36, and 72-month,incorporatinghistology type, chemotherapy, and regional nodes (Fig. 5A). A high score on the nomogram indicated a higher risk of SPM and a lower risk of death. The performance of the nomogram was evaluated using ROC curves in the training and testing sets (Fig. 5B and C). The AUC (95% CI) for the 24-, 36-, and 72-month survival rates were0.633 (0.575–0.691), 0.633 (0.577–0.686), and 0.701 (0.639–0.764) in the training set, and 0.632 (0.534–0.729), 0.658 (0.581–0.735), and 0.734 (0.646–0.823) in the testing set. Calibration analysis (Fig. 5D and E) was performed to compare the predicted SPM probabilities from the nomogram with the observed probabilities at 24, 36, and 72-months in both the training and testing set. The nomogram calibration plot indicates that the nomogram was well calibrated, with the similar predicted probabilities in the training and testing sets compared to an ideal model. Using a decision curve analysis (DCA), the nomogram for 24-, 48- 72-month SPM have exhibited a wide range of thresholds probabilities for net clinical benefit (Fig. 5F–K). The C-index and Brier score for the 72 months cutoff were 0.685 and 0.09 in the training set, and were confirmed to be 0.734 and 0.08 in the testing set, respectively (Supplementary Table S4). These results demonstrate that the nomogram exhibits good discriminative ability and accurate predictive performance.
Competing-risk nomogram predicting 24-, 48- and 72-month cumulative incidence probabilities of SPMs for GC patients based on training cohort. The nomogram is used by adding up the points identified on the points scale for each variable. The total points projected on the bottom scales indicate the probabilities of 24-, 48- and 72-month SPM (A). ROC calibration plots (B,C) and calibration curves (D,E) of SPM at 24-, 48- and 72-months in the training set and validation set. Decision curve analysis of the model for predicting 24-, 48- and 72-month SPM in the training set (F–H) and test set (I–K). ACs, adenocarcinomas; SRCC, signet ring cell carcinoma.
Discussion
No relevant previous studies have focused on the risk factors of malignant tumours secondary to specifically early gastric cancer24,25, our analysis includes many characteristics, such as demographic characteristics and AJCC stages. This research has potential applications and research prospects in helping clinical doctors and researchers to better understand the mechanisms and risk factors related to SPM, thus guiding early diagnosis and treatment. We conducted a COX analysis of related factors and obtained some new findings that did not cover in previous studies, such as the discovery of marital status influencing gastric cancer SPM over time, the increased incidence of SPM due to divorce or separation, and the discovery that surgery may increase the risk of SPM, indicating the relative risk of specific surgical methods (subtotal/total resection and biopsy/local excision relative to the risk of no surgery and other surgeries). In addition, based on the confirmation that chemotherapy is an independent risk factor for SPM, we identify that increased age, widowed, AJCC IB stage, and surgical biopsy/local excision treatment positively correlated to SPMs, whereas radiotherapy was negatively correlated to SPMs. Through the above analysis, we can assist in identifying high-risk patients who are susceptible to SPM and provide them with personalized treatment plans to improve their prognosis and survival.
The incidence of SPM in gastric cancer patients has been observed increase in several pieces of research. In a large hospital-based study of patients who underwent abdominopelvic CT scans and were thus at high risk of intra-abdominal SPM in patients with early gastric cancer26. The incidence of extra-gastric recurrence was ranged from 1.4 to 2.2% herein slightly higher among patients after surgical resection of EGC, than that after endoscope resection27. In our study, 155 of 3289 (4.7%) GC patients developed SPM, with an incidence comparable to that of other similar studies. The improvement of therapeutic level has extended the life of GC patients. This special patient group has a range of potential health issues; many cancer survivors are at risk of creating a second primary malignancy. SPM is portends a poor prognosis with the median survival time is usually around one year28. Therefore, it is particularly important to rationally standardize the future treatment regimens, clear the excessive risk elements for the occurrence of SPMs, and boost centralized intervention and clinical interventions.
Studies that have found risk factors for SPM of GC include male, old age (over 70 years), with another basic diseases including COPD, diabetes, cirrhosis. In addition, chemotherapy and radiotherapy are independent risk factors, but surgery is a protective factor29. Some recent studies on the effects of patient characteristics have yielded conflicting results. For example, young adults have a higher risk of SPM than older survivors and being male is not a risk factors30. According to KM analysis in our study, AC and SRCC produce more SPM than other types of gastric cancer. The main anatomical parts of GC, such as curvature, produce lower SPM than other sites, and with the increase in lymph nodes region and divorce/separation also increases SPM. Unlike previous findings, in our Multivariate cox regression analysis, SPM was affected only by histological types of GC. If considering death as a competitive factor, the histological types and therapy methods, including chemotherapy and surgical, are independent risk factors of SPM. Biopsy/local excision is associated with a low risk of SPM, whereas subtotal and total resection with high risk. Therefore, It is important to closely monitor GC patients and establish follow-up programs to detect SPMs29. In the present study, we report that patients in the early AJCC stage, T1 stage and no lymph node infiltration, without irradiation and chemotherapy, had an increased risk of developing SPMs than in those who in the distant stage and received radio- and chemotherapy. One possible explanation is that patients in the early stage generally have a better prognosis and longer follow-up time, increasing the likelihood of developing SPMs. In contrast, patients in the advanced stage may have limited follow-up time and could be lost to follow-up, resulting in a lower chance of developing SPMs31. Further investigations through randomized controlled trials are required to clarify the impact of radio- and chemotherapy.
Some studies have shown that use of chemotherapy not associated with incidence of SPM32, which is inconsistent with our study. The cause may be linked to the dose of the chemotherapy drug used33 or whether the statistical results came from a randomized controlled trial34. Clinicians may be aware of tips from our results to enable them give patient optimal adjuvant therapy but not overzealous chemotherapy. While the SEER database lacks detailed data, it is worth noting that chemotherapy may still carry a higher risk of certain SPMs compared to radiotherapy, albeit to a lesser extent35. However, it is important to highlight that limited data exist thus far regarding the specific risk of chemotherapy-associated SPMs36. Furthermore, it is expected that the use of ICIs and immune-based combinations is destined to grow in gastric cancer patients in the future. While ICIs monotherapy and immune-based combinations are generally regarded as having an acceptable safety profile, it is important to note that ICIs can cause a distinct set of treatment-related adverse events. These adverse events, commonly referred to as immune-related adverse events (irAEs)9, there is a pressing need for additional studies to comprehensively understand the implications of these events. Unlike previous studies that examined the general probability of developing SPMs among cancer survivors, we have established a competing-risk nomogram37 to identify GC survivors who are at a high risk of developing SPMs. This nomogram allows us to estimate the likelihood of SPMs based onpatients’ characteristics. To assess the accuracy and reliability of our model predictions, we utillized measures such as AUC values, C indexes, calibration curves, and DCA test line plots. The results indicate that our nomogram performed well and can be generalized without limitations based on race, gender, or marital status.
Currently, there is a knowledge gap in this research field, primarily in the following areas. First, there is a lack of research on the mechanisms of SPM, which requires further exploration in basic research. Second, the identification and validation of high-risk factors for SPM require larger-scale clinical studies and validation38. To address these knowledge gaps, researchers should actively conduct molecular biology and clinical oncology, explore the mechanisms of SPM through interdisciplinary collaborations, and conduct large-scale clinical cohort studies. Meanwhile, we should still acknowledge some limitations in this study. First, the SEER dataset lacks data on doses of radiotherapy, specifics on chemotherapy regimens and genetic mutations, and we have no access to patient-specific lifestyle data, including smoking and alcohol intake, as this information was not included in the database. Second, the low rate of SPM limited statistical power for the analysis, specifically for the stratified analysis. Third, we could not validate the constructed model using other cohorts due to the disease’s rarity. Finally, as this study utilized a retrospective methodology, it is necessary to conduct additional prospective studies to determine if the results can be generalized for predicting SPMs before clinical application. Furthermore, since the number of participants in this study was not adequate to enable multivariate analyses to produce more accurate and general conclusions, more careful evaluation in a large sample size will be required in the future.
This study presented a comprehensive analysis of patient characteristics and predictors of subsequent SPMs based on a large population. These findings suggested that in specific histologic types of GC, the risk of SPM increases when areas of lymph node invasion are missed after local surgical resection and concurrent chemotherapy. Over the next five years, we believe that the development of this field will primarily focus on the following aspects. Firstly, in order to enhance early screening and prevention, further research should be conducted on the mechanism and prediction model of SPM. The assessment of SPM risk in patients with GC should also take into consideration crucial factors like inflammation, inflammation-driven gastric cancer and tumour microenvironment (TME)39, and the nutritional status of patients. The collection of peripheral blood-based biomarkers over an extended period can serve as an indirect indicator of patient immune status and effective measure to evaluate SPM in the future. Examples of these biomarkers include the neutrophil–lymphocyte ratio (NLR), the platelet-lymphocyte ratio (PLR), the pan-immune-inflammation value, the albumin levels and the Royal Marsden Hospital (RMH) score. Recent studies have demonstrated that these biomarkers can serve as valuable prognostic tools, not only for patient selection in clinical trials but also for patients in real-world clinical settings40,41. Secondly, exploring treatment strategies for SPM, including surgical method selection, radiotherapy and chemotherapy regimens, and the use of targeted drugs and ICIs as adjuvant therapy, to improve treatment outcomes and survival rates for patients. Lastly, integrating new technologies such as artificial intelligence and big data, research on personalized treatment plans based on clinical characteristics and genetic information. In conclusion, future research endeavors will continue to enhance the prediction and intervention strategies of SPM, ensuring its optimum application in clinical practice and decision-making for the treatment of GC.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files]. The raw data was downloaded from the Surveillance, Epidemiology, and End Results (SEER) application of the United States National Cancer Institute (https://seer.cancer.gov/) and submitted to the supplementary materials.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Park, J. et al. Prediction of the indication criteria for endoscopic resection of early gastric cancer. World J. Gastroenterol. 21, 11160–11167. https://doi.org/10.3748/wjg.v21.i39.11160 (2015).
Guo, L. et al. Determinants of participation and detection rate of upper gastrointestinal cancer from population-based screening program in China. Cancer Med. 8, 7098–7107. https://doi.org/10.1002/cam4.2578 (2019).
The Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J. Gastric Cancer 16, 131–140. https://doi.org/10.5230/jgc.2016.16.3.131 (2016).
Kim, H. et al. Effect of laparoscopic distal gastrectomy vs open distal gastrectomy on long-term survival among patients with stage I gastric cancer: The KLASS-01 randomized clinical trial. JAMA Oncol. 5, 506–513. https://doi.org/10.1001/jamaoncol.2018.6727 (2019).
Li, Y., Zhu, Z., Ma, F., Xue, L. & Tian, Y. Improving survival of stage II-III primary gastric signet ring cell carcinoma by adjuvant chemoradiotherapy. Cancer Med. 9, 6617–6628. https://doi.org/10.1002/cam4.3342 (2020).
Angela Dalia, R., Alessandro, R. & Giovanni, B. DNA damage response alterations in gastric cancer: Knocking down a new wall. Futur. Oncol. 17, 865. https://doi.org/10.2217/fon-2020-0989 (2021).
Ricci, A. D. et al. Novel HER2-directed treatments in advanced gastric carcinoma: AnotHER paradigm shift?. Cancers (Basel) 13, 1664. https://doi.org/10.3390/cancers13071664 (2021).
Alessandro, R. et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: The MOUSEION-05 study. Cancer Immunol. Immunother. 72, 1381. https://doi.org/10.1007/s00262-023-03366-x (2023).
Giuseppe, V. et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: A systematic review and meta-analysis. Eur. J. Cancer 177, 175. https://doi.org/10.1016/j.ejca.2022.09.031 (2022).
Wood, M. et al. Second malignant neoplasms: Assessment and strategies for risk reduction. J. Gastric Cancer 30, 3734–3745. https://doi.org/10.1200/jco.2012.41.8681 (2012).
Kim, C. et al. Prediction of metachronous multiple primary cancers following the curative resection of gastric cancer. BMC Cancer 13, 394. https://doi.org/10.1186/1471-2407-13-394 (2013).
Choi, G. et al. Genetically mediated Nf1 loss in mice promotes diverse radiation-induced tumors modeling second malignant neoplasms. Cancer Res. 72, 6425–6434. https://doi.org/10.1158/0008-5472.Can-12-1728 (2012).
Niimoto, M. et al. Secondary malignancies after gastrectomy for gastric cancer. Gan to kagaku ryoho Cancer Chemother. 13, 1475–1483 (1986).
Li, S., Luo, Y., Hou, Q., Chu, H. & Zheng, H. J. Incidence features of second primary malignancy among gastric cancer survivors, 1992-2012. Transl. Cancer Res. 9, 7001–7011. https://doi.org/10.21037/tcr-20-1105 (2020).
Shah, B., Khanal, A. & Hewett, Y. J. Second primary malignancies in adults with gastric cancer—A US population-based study. Front. Oncol. 6, 82. https://doi.org/10.3389/fonc.2016.00082 (2016).
Yang, J. et al. Brief introduction of medical database and data mining technology in big data era. J. Evid. Based Med. 13, 57–69. https://doi.org/10.1111/jebm.12373 (2020).
Muir, C. & Percy, C. J. Cancer Registration: Principles and Methods. In Classification and coding of neoplasms 64–81 (IARC Scientific Publications, 1991).
Adjei Boakye, E. et al. Trends in the risk and burden of second primary malignancy among survivors of smoking-related cancers in the United States. Int. J. Cancer 145, 143–153. https://doi.org/10.1002/ijc.32101 (2019).
Scrucca, L., Santucci, A. & Aversa, F. Regression modeling of competing risk using R: An in depth guide for clinicians. Bone Marrow Transpl. 45, 1388–1395. https://doi.org/10.1038/bmt.2009.359 (2010).
Alba, A. et al. Discrimination and calibration of clinical prediction models: Users’ guides to the medical literature. JAMA 318, 1377–1384. https://doi.org/10.1001/jama.2017.12126 (2017).
Wolbers, M., Koller, M., Witteman, J. & Steyerberg, E. J. E. Prognostic models with competing risks: Methods and application to coronary risk prediction. Epidemiology 20, 555–561. https://doi.org/10.1097/EDE.0b013e3181a39056 (2009).
Fitzgerald, M., Saville, B. & Lewis, R. J. J. Decision curve analysis. JAMA 313, 409–410. https://doi.org/10.1001/jama.2015.37 (2015).
Cho, C. et al. The incidence and locational predilection of metachronous tumors after endoscopic resection of high-grade dysplasia and early gastric cancer. Surg. Endosc. 31, 389–397. https://doi.org/10.1007/s00464-016-4985-8 (2017).
Yang, Y., Yang, Y. & Yan, S. Risk and survival of second primary malignancies following diagnosis of gastric mucosa-associated lymphoid tissue lymphomas: A population-based study. Curr. Probl. Cancer 45, 100735. https://doi.org/10.1016/j.currproblcancer.2021.100735 (2021).
Kim, T. et al. Risk of second primary malignancies among patients with early gastric cancer exposed to recurrent computed tomography scans. Cancers 13, 1144. https://doi.org/10.3390/cancers13051144 (2021).
Youn, H. et al. Recurrence after curative resection of early gastric cancer. Ann. Surg. Oncol. 17, 448–454. https://doi.org/10.1245/s10434-009-0772-2 (2010).
Zheng, X. et al. Second primary malignancies among cancer patients. Ann. Transl. Med. 8, 638. https://doi.org/10.21037/atm-20-2059 (2020).
Kim, J., Jang, J., Chang, Y. & Kim, Y. Clinical features of second primary cancers arising in early gastric cancer patients after endoscopic resection. World J. Gastroenterol. 21, 8358–8365. https://doi.org/10.3748/wjg.v21.i27.8358 (2015).
Lee, J. et al. Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer 122, 116–123. https://doi.org/10.1002/cncr.29685 (2016).
Zhang, B. et al. Risk of second primary malignancies in colon cancer patients treated with colectomy. Front. Oncol. 10, 1154. https://doi.org/10.3389/fonc.2020.01154 (2020).
Wang, T., Liu, C., Chao, T., Chen, T. & Hu, Y. Second primary malignancy risk after radiotherapy in rectal cancer survivors. World J. Gastroenterol. 24, 4586–4595. https://doi.org/10.3748/wjg.v24.i40.4586 (2018).
Maloney, S. et al. Induction of thrombospondin-1 partially mediates the anti-angiogenic activity of dexrazoxane. Br. J. Cancer 101, 957–966. https://doi.org/10.1038/sj.bjc.6605203 (2009).
Holstein, S. et al. Updated analysis of CALGB (Alliance) 100104 assessing lenalidomide versus placebo maintenance after single autologous stem-cell transplantation for multiple myeloma: A randomised, double-blind, phase 3 trial. Lancet Haematol. 4, e431–e442. https://doi.org/10.1016/s2352-3026(17)30140-0 (2017).
Kier, M. et al. Second malignant neoplasms and cause of death in patients with germ cell cancer: A Danish Nationwide cohort study. JAMA Oncol. 2, 1624–1627. https://doi.org/10.1001/jamaoncol.2016.3651 (2016).
Turcotte, L. et al. Chemotherapy and risk of subsequent malignant neoplasms in the childhood cancer survivor study cohort. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 37, 3310–3319. https://doi.org/10.1200/jco.19.00129 (2019).
Jia, H., Li, Q., Yuan, J., Sun, X. & Wu, Z. Second primary malignancies in patients with colorectal cancer: A population-based analysis. Oncol. 25, e644–e650. https://doi.org/10.1634/theoncologist.2019-0266 (2020).
Alessandro, R. et al. Third- and later-line treatment in advanced or metastatic gastric cancer: A systematic review and meta-analysis. Futur. Oncol. 16, 4409. https://doi.org/10.2217/fon-2019-0429 (2019).
Karim, R. et al. Tumor-associated macrophages and inflammatory microenvironment in gastric cancer: Novel translational implications. Int. J. Mol. Sci. 22, 3805. https://doi.org/10.3390/ijms22083805 (2021).
Deniz Can, G. et al. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front. Mol. Biosci. 9, 1039121. https://doi.org/10.3389/fmolb.2022.1039121 (2022).
Taha Koray, S., Alessandro, R., Sercan, A. & Deniz Can, G. Prognostic significance of the Royal Marsden Hospital (RMH) score in patients with cancer: A systematic review and meta-analysis. Cancers (Basel) 16, 1835. https://doi.org/10.3390/cancers16101835 (2024).
Acknowledgements
The authors would like to express their gratitude to the two anonymous referees for their valuable comments and thorough review of the manuscript. This research was supported by grants from Natural Science Foundation of Gansu Province (21JR11RA016, 22JR11RA237), the Fundamental Research Funds for the Central Universities of the Northwest Minzu University (31920220113, 31920220114, 31920230002) and the National Natural Science Foundation of China (NSFC) (81860716).
Funding
This work was supported by funding from Natural Science Foundation of Gansu Province (21JR11RA016, 22JR11RA237), the Fundamental Research Funds for the Central Universities of the Northwest Minzu University (31920220113, 31920220114, 31920230002) and the National Natural Science Foundation of China (NSFC) (81860716).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Fei Zhao, Lijing Zhang, Zhifang Zhao, Long Jin, Yu Zhao and Jin Zhao. The first draft of the manuscript was written by Lei Song and all authors commented on previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, L., Zhao, F., Zhang, L. et al. Analyzing risk factors for second malignancies in early gastric carcinoma from the SEER database. Sci Rep 14, 17761 (2024). https://doi.org/10.1038/s41598-024-68776-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-68776-y
- Springer Nature Limited