Abstract
Background
The antitumor efficacy of immune checkpoint inhibitors (ICIs) has increasingly emerged during the last few years. However, there is a need to identify the safety profile of these agents more comprehensively, including liver toxicity.
Materials and methods
Herein, we performed a meta-analysis to assess the risk of all-grade and grade 3–4 hypertransaminasemia in cancer patients receiving ICIs—as monotherapy or in combination with other anticancer agents. All the relevant trials were retrieved through EMBASE, Cochrane Library, and PubMed/Medline databases; eligible studies were selected according to PRISMA statement. The pooled relative risk (RR) and 95% confidence interval (CI) were extracted.
Results
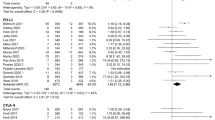
Fifty-nine studies were included. The pooled RRs for all-grade AST and ALT increase were 1.45 (95% CI 1.26–1.67) (Supplementary Fig. 3) and 1.51 (95% CI 1.29–1.77) in patients receiving ICIs monotherapy and immune-based combinations compared to control treatment, respectively. The pooled RRs for grade 3–4 AST and ALT increase were 2.16 (95% CI 1.77–2.64) and 2.3 (95% CI 1.91–2.77).
Conclusions
According to our results, ICIs monotherapy and immune-based combinations were associated with higher risk of all-grade and grade 3–4 hypertransaminasemia. Monitoring liver function should be recommended in cancer patients treated with ICIs monotherapy or immune-based combination, and in case of underlying liver disease, a careful risk–benefit assessment appears as a mandatory need.


Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660. (Epub 2021 Feb 4 PMID: 33538338)
Zhang Y, Zhang Z (2020) The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol 17(8):807–821. https://doi.org/10.1038/s41423-020-0488-6
Xie J, Fu L, Jin L (2021) Immunotherapy of gastric cancer: past, future perspective and challenges. Pathol Res Pract 218:153322. https://doi.org/10.1016/j.prp.2020.153322
Mollica V, Santoni M, Matrana MR, Basso U, De Giorgi U, Rizzo A, Maruzzo M, Marchetti A, Rosellini M, Bleve S, Maslov D, Tawagi K, Philon E, Blake Z, Massari F (2022) Concomitant proton pump inhibitors and outcome of patients treated with nivolumab alone or plus ipilimumab for advanced renal cell carcinoma. Target Oncol 17(1):61–68. https://doi.org/10.1007/s11523-021-00861-y. (Epub 2021 Dec 11 PMID: 34894318)
Rosellini M, Marchetti A, Mollica V, Rizzo A, Santoni M, Massari F (2022) Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat Rev Urol. https://doi.org/10.1038/s41585-022-00676-0
Yang Y (2015) Cancer immunotherapy: harnessing the immune system to battle cancer. J Clin Invest 125(9):3335–3337. https://doi.org/10.1172/JCI83871
Riley RS, June CH, Langer R, Mitchell MJ (2019) Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov 18(3):175–196. https://doi.org/10.1038/s41573-018-0006-z.PMID:30622344;PMCID:PMC6410566
Viscardi G, Tralongo AC, Massari F, Lambertini M, Mollica V, Rizzo A, Comito F, Di Liello R, Alfieri S, Imbimbo M, Della Corte CM, Morgillo F, Simeon V, Lo Russo G, Proto C, Prelaj A, De Toma A, Galli G, Signorelli D, Ciardiello F, Remon J, Chaput N, Besse B, de Braud F, Garassino MC, Torri V, Cinquini M, Ferrara R (2022) Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer 177:175–185. https://doi.org/10.1016/j.ejca.2022.09.031. (Epub 2022 Oct 5 PMID: 36368251)
Sangro B, Sarobe P, Hervás-Stubbs S, Melero I (2021) Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 18(8):525–543. https://doi.org/10.1038/s41575-021-00438-0
Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX, Finn RS (2022) Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol 19(3):151–172. https://doi.org/10.1038/s41571-021-00573-2. (Epub 2021 Nov 11 PMID: 34764464)
Cuevas LM, Daud AI (2018) Immunotherapy for melanoma. Semin Cutan Med Surg 37(2):127–131. https://doi.org/10.12788/j.sder.2018.028. (PMID: 30040090)
Ruiz-Cordero R, Devine WP (2020) Targeted therapy and checkpoint immunotherapy in lung cancer. Surg Pathol Clin 13(1):17–33. https://doi.org/10.1016/j.path.2019.11.002. (PMID: 32005431)
Reck M, Remon J, Hellmann MD (2022) First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol 40(6):586–597. https://doi.org/10.1200/JCO.21.01497
Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, Battelli N, Massari F (2022) The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: the MOUSEION-01 study. Crit Rev Oncol Hematol 170:103596. https://doi.org/10.1016/j.critrevonc.2022.103596
Rizzo A, Santoni M, Mollica V, Logullo F, Rosellini M, Marchetti A, Faloppi L, Battelli N, Massari F (2021) Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert Opin Drug Metab Toxicol 17(12):1455–1466. https://doi.org/10.1080/17425255.2021.2029405. (Epub 2022 Jan 24 PMID: 35029519)
Marrone KA, Ying W, Naidoo J (2016) Immune-related adverse events from immune checkpoint inhibitors. Clin Pharmacol Ther 100(3):242–251
Suzman DL, Pelosof L, Rosenberg A, Avigan MI (2018) Hepatotoxicity of immune checkpoint inhibitors: an evolving picture of risk associated with a vital class of immunotherapy agents. Liver Int 38(6):976–987
Wang W, Lie P, Guo M, He J (2017) Risk of hepatotoxicity in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis of published data. Int J Cancer 141(5):1018–1028
Puzanov I, Diab A, Abdallah K et al (2017) Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the society for immunotherapy of cancer (SITC) toxicity management working group. J Immunother Cancer 5:95
Champiat S, Lambotte O, Barreau E et al (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27(4):559–574
Peeraphatdit TB, Wang J, Odenwald MA, Hu S, Hart J, Charlton MR (2020) Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology 72(1):315–329
Tian Y, Abu-Sbeih H, Wang Y (2020) Immune checkpoint inhibitors-induced hepatitis. Adv Exp Med Biol 995:159–164
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) PRISMA Group preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535
Higgins JPT, Thomas J, Chandler J et al (2019) Cochrane hand-book for systematic reviews of interventions, 2nd edn. John Wiley and Sons, Chichester (UK)
Powles T, van der Heijden MS, Castellano D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL, De Giorgi U, Bögemann M, Bamias A, Eigl BJ, Gurney H, Mukherjee SD, Fradet Y, Skoneczna I, Tsiatas M, Novikov A, Suárez C, Fay AP, Duran I, Necchi A, Wildsmith S, He P, Angra N, Gupta AK, Levin W, Bellmunt J (2020) DANUBE study investigators. Durvalumab alone and durvalumab plus tremelimumab versus chemotherapy in previously untreated patients with unresectable, locally advanced or metastatic urothelial carcinoma (DANUBE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol 21(12):1574–1588 https://doi.org/10.1016/S1470-2045(20)30541-6
Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Każarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Thiyagarajah P, Jiang H, Paz-Ares L (2021) CASPIAN investigators. durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 22(1):51–65. https://doi.org/10.1016/S1470-2045(20)30539-8
Renouf DJ, Loree JM, Knox JJ, Topham JT, Kavan P, Jonker D, Welch S, Couture F, Lemay F, Tehfe M, Harb M, Aucoin N, Ko YJ, Tang PA, Ramjeesingh R, Meyers BM, Kim CA, Du P, Jia S, Schaeffer DF, Gill S, Tu D, O’Callaghan CJ (2022) The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat Commun 26 13(1):5020. https://doi.org/10.1038/s41467-022-32591-8
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330. https://doi.org/10.1056/NEJMoa1412082. (Epub 2014 Nov 16 PMID: 25399552)
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373(17):1627–1639. https://doi.org/10.1056/NEJMoa1507643
Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Arén Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373(2):123–135. https://doi.org/10.1056/NEJMoa1504627
Larkin J, Minor D, D’Angelo S, Neyns B, Smylie M, Miller WH Jr, Gutzmer R, Linette G, Chmielowski B, Lao CD, Lorigan P, Grossmann K, Hassel JC, Sznol M, Daud A, Sosman J, Khushalani N, Schadendorf D, Hoeller C, Walker D, Kong G, Horak C, Weber J (2018) Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in checkmate 037: a randomized, controlled, open-label phase iii trial. J Clin Oncol 36(4):383–390. https://doi.org/10.1200/JCO.2016.71.8023
Gillison ML, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Li L, Ferris RL (2018) CheckMate 141: 1-year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist 23(9):1079–1082. https://doi.org/10.1634/theoncologist.2017-0674
Cella D, Grünwald V, Escudier B, Hammers HJ, George S, Nathan P, Grimm MO, Rini BI, Doan J, Ivanescu C, Paty J, Mekan S, Motzer RJ (2019) Patient-reported outcomes of patients with advanced renal cell carcinoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol 20(2):297–310. https://doi.org/10.1016/S1470-2045(18)30778-2
Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, Zhang L, Tu HY, Wu L, Feng J, Zhang Y, Luft AV, Zhou J, Ma Z, Lu Y, Hu C, Shi Y, Baudelet C, Cai J, Chang J (2019) Nivolumab versus docetaxel in a predominantly chinese patient population with previously treated advanced nsclc: checkmate 078 randomized phase iii clinical trial. J Thorac Oncol 14(5):867–875. https://doi.org/10.1016/j.jtho.2019.01.006. (Epub 2019 Jan 17 PMID: 30659987)
Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr O, Phuphanich S, Sepulveda JM, De Souza P, Sahebjam S, Carleton M, Tatsuoka K, Taitt C, Zwirtes R, Sampson J, Weller M (2020) Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: the checkmate 143 phase 3 randomized clinical trial. JAMA Oncol 6(7):1003–1010. https://doi.org/10.1001/jamaoncol.2020.1024.PMID:32437507;PMCID:PMC7243167
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, Liu T, Schenker M, Yanez P, Tehfe M, Kowalyszyn R, Karamouzis MV, Bruges R, Zander T, Pazo-Cid R, Hitre E, Feeney K, Cleary JM, Poulart V, Cullen D, Lei M, Xiao H, Kondo K, Li M, Ajani JA (2021) First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398(10294):27–40. https://doi.org/10.1016/S0140-6736(21)00797-2
Choueiri TK, Powles T, Burotto M, Escudier B, Bourlon MT, Zurawski B, Oyervides Juárez VM, Hsieh JJ, Basso U, Shah AY, Suárez C, Hamzaj A, Goh JC, Barrios C, Richardet M, Porta C, Kowalyszyn R, Feregrino JP, Żołnierek J, Pook D, Kessler ER, Tomita Y, Mizuno R, Bedke J, Zhang J, Maurer MA, Simsek B, Ejzykowicz F, Schwab GM, Apolo AB, Motzer RJ (2021) CheckMate 9ER investigators nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384(9):829–841. https://doi.org/10.1056/NEJMoa2026982
Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S, Matsumoto K, Ito K, Mandai M, Nakai H, Sakuragi N, Watari H, Takahashi N, Kato H, Hasegawa K, Yonemori K, Mizuno M, Takehara K, Niikura H, Sawasaki T, Nakao S, Saito T, Enomoto T, Nagase S, Suzuki N, Matsumoto T, Kondo E, Sonoda K, Aihara S, Aoki Y, Okamoto A, Takano H, Kobayashi H, Kato H, Terai Y, Takazawa A, Takahashi Y, Namba Y, Aoki D, Fujiwara K, Sugiyama T, Konishi I (2021) Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in japan (NINJA). J Clin Oncol 39(33):3671–3681. https://doi.org/10.1200/JCO.21.00334
Hayashi H, Sugawara S, Fukuda Y, Fujimoto D, Miura S, Ota K, Ozawa Y, Hara S, Tanizaki J, Azuma K, Omori S, Tachihara M, Nishino K, Bessho A, Chiba Y, Haratani K, Sakai K, Nishio K, Yamamoto N, Nakagawa K (2022) A randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res 28(5):893–902. https://doi.org/10.1158/1078-0432.CCR-21-3194. (PMID: 34921023)
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA (2018) IMpassion130 trial investigators atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121. https://doi.org/10.1056/NEJMoa1809615
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M (2018) IMpower150 study group atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301. https://doi.org/10.1056/NEJMoa1716948
Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, Oudard S, Retz MM, Castellano D, Bamias A, Fléchon A, Gravis G, Hussain S, Takano T, Leng N, Kadel EE 3rd, Banchereau R, Hegde PS, Mariathasan S, Cui N, Shen X, Derleth CL, Green MC, Ravaud A (2018) Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 391(10122):748–757. https://doi.org/10.1016/S0140-6736(17)33297-X
Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, Falcone A, Fakih M, Kozloff M, Segal NH, Sobrero A, Yan Y, Chang I, Uyei A, Roberts L, Ciardiello F (2019) IMblaze370 investigators atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 20(6):849–861. https://doi.org/10.1016/S1470-2045(19)30027-0
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, Zer A, Reinmuth N, Sadiq A, Sandler A, Lin W, Ochi Lohmann T, Archer V, Wang L, Kowanetz M, Cappuzzo F (2019) Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20(7):924–937. https://doi.org/10.1016/S1470-2045(19)30167-6. (Epub 2019 May 20 PMID: 31122901)
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL (2020) IMbrave150 investigators atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382(20):1894–1905. https://doi.org/10.1056/NEJMoa1915745
Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, Koehler A, Sohn J, Iwata H, Telli ML, Ferrario C, Punie K, Penault-Llorca F, Patel S, Duc AN, Liste-Hermoso M, Maiya V, Molinero L, Chui SY, Harbeck N (2020) Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396(10257):1090–1100. https://doi.org/10.1016/S0140-6736(20)31953-X. (Epub 2020 Sep 20 PMID: 32966830)
Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, Morise M, Felip E, Andric Z, Geater S, Özgüroğlu M, Zou W, Sandler A, Enquist I, Komatsubara K, Deng Y, Kuriki H, Wen X, McCleland M, Mocci S, Jassem J, Spigel DR (2020) Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med 383(14):1328–1339. https://doi.org/10.1056/NEJMoa1917346. (PMID: 32997907)
Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A, Kenmotsu H, Chen YM, Chella A, Sugawara S, Voong D, Wu F, Yi J, Deng Y, McCleland M, Bennett E, Gitlitz B, Wakelee H (2021) IMpower010 investigators adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 398(10308):1344–1357. https://doi.org/10.1016/S0140-6736(21)02098-5
Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz M, Degaonkar V, Shi Y, Mariathasan S, Grivas P, Drakaki A, O’Donnell PH, Rosenberg JE, Geynisman DM, Petrylak DP, Hoffman-Censits J, Bedke J, Kalebasty AR, Zakharia Y, van der Heijden MS, Sternberg CN, Davarpanah NN, Powles T (2021) IMvigor010 study group adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 22(4):525–537. https://doi.org/10.1016/S1470-2045(21)00004-8
Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, Semiglazov V, Reinisch M, Patel S, Patre M, Morales L, Patel SL, Kaul M, Barata T, O’Shaughnessy J (2021) IMpassion131 investigators primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 32(8):994–1004. https://doi.org/10.1016/j.annonc.2021.05.801
Cho BC, Abreu DR, Hussein M, Cobo M, Patel AJ, Secen N, Lee KH, Massuti B, Hiret S, Yang JCH, Barlesi F, Lee DH, Ares LP, Hsieh RW, Patil NS, Twomey P, Yang X, Meng R, Johnson ML (2022) Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol 23(6):781–792. https://doi.org/10.1016/S1470-2045(22)00226-1. (Epub 2022 May 13 PMID: 35576957)
Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T (2022) Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 23(8):995–1008. https://doi.org/10.1016/S1470-2045(22)00326-6. (Epub 2022 Jul 4 PMID: 35798016)
Mettu NB, Ou FS, Zemla TJ, Halfdanarson TR, Lenz HJ, Breakstone RA, Boland PM, Crysler OV, Wu C, Nixon AB, Bolch E, Niedzwiecki D, Elsing A, Hurwitz HI, Fakih MG, Bekaii-Saab T (2022) Assessment of capecitabine and bevacizumab with or without atezolizumab for the treatment of refractory metastatic colorectal cancer: a randomized clinical trial. JAMA Netw Open 5(2):e2149040. https://doi.org/10.1001/jamanetworkopen.2021.49040
Huober J, Barrios CH, Niikura N, Jarząb M, Chang YC, Huggins-Puhalla SL, Pedrini J, Zhukova L, Graupner V, Eiger D, Henschel V, Gochitashvili N, Lambertini C, Restuccia E, Zhang H (2022) IMpassion050 trial investigators. atezolizumab with neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2-positive early breast cancer: primary results of the randomized phase iii impassion050 trial. J Clin Oncol 40(25):2946–2956. https://doi.org/10.1200/JCO.21.02772
Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, Albiges L, Rini B, Tomita Y, Kann AG, Procopio G, Massari F, Zibelman M, Antonyan I, Huseni M, Basu D, Ci B, Leung W, Khan O, Dubey S, Bex A (2022) Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet 400(10358):1103–1116. https://doi.org/10.1016/S0140-6736(22)01658-0. (Epub 2022 Sep 10 PMID: 36099926)
Bang YJ, Ruiz EY, Van Cutsem E, Lee KW, Wyrwicz L, Schenker M, Alsina M, Ryu MH, Chung HC, Evesque L, Al-Batran SE, Park SH, Lichinitser M, Boku N, Moehler MH, Hong J, Xiong H, Hallwachs R, Conti I, Taieb J (2018) Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: primary analysis of JAVELIN Gastric 300. Ann Oncol 29(10):2052–2060. https://doi.org/10.1093/annonc/mdy264.PMID:30052729;PMCID:PMC6225815
Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, Özgüroğlu M, Szczesna A, Polychronis A, Uslu R, Krzakowski M, Lee JS, Calabrò L, Arén Frontera O, Ellers-Lenz B, Bajars M, Ruisi M, Park K (2018) Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 19(11):1468–1479. https://doi.org/10.1016/S1470-2045(18)30673-9
Motzer RJ, Penkov K, Haanen J, Rini B, Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S, Uemura M, Lee JL, Vasiliev A, Miller WH Jr, Gurney H, Schmidinger M, Larkin J, Atkins MB, Bedke J, Alekseev B, Wang J, Mariani M, Robbins PB, Chudnovsky A, Fowst C, Hariharan S, Huang B, di Pietro A, Choueiri TK (2019) Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380(12):1103–1115. https://doi.org/10.1056/NEJMoa1816047
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén A, Loriot Y, Sridhar SS, Tsuchiya N, Kopyltsov E, Sternberg CN, Bellmunt J, Aragon-Ching JB, Petrylak DP, Laliberte R, Wang J, Huang B, Davis C, Fowst C, Costa N, Blake-Haskins JA, di Pietro A, Grivas P (2020) Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 383(13):1218–1230. https://doi.org/10.1056/NEJMoa2002788. (Epub 2020 Sep 18 PMID: 32945632)
Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, Cubillo Gracian A, Takano T, Wong R, Safran H, Vaccaro GM, Wainberg ZA, Silver MR, Xiong H, Hong J, Taieb J, Bang YJ (2021) Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol 39(9):966–977. https://doi.org/10.1200/JCO.20.00892
Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, Harrington K, Chang PM, Lin JC, Razaq MA, Teixeira MM, Lövey J, Chamois J, Rueda A, Hu C, Dunn LA, Dvorkin MV, De Beukelaer S, Pavlov D, Thurm H, Cohen E (2021) Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol 22(4):450–462. https://doi.org/10.1016/S1470-2045(20)30737-3. (PMID: 33794205)
Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, Richardson GE, Sessa C, Yonemori K, Banerjee S, Leary A, Tinker AV, Jung KH, Madry R, Park SY, Anderson CK, Zohren F, Stewart RA, Wei C, Dychter SS, Monk BJ (2021) Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol 22(7):1034–1046. https://doi.org/10.1016/S1470-2045(21)00216-3. (Epub 2021 Jun 15 PMID: 34143970)
Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L, Poddubskaya EV, Scambia G, Shparyk YV, Lim MC, Bhoola SM, Sohn J, Yonemori K, Stewart RA, Zhang X, Perkins Smith J, Linn C, Ledermann JA (2021) Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial. Lancet Oncol 22(9):1275–1289. https://doi.org/10.1016/S1470-2045(21)00342-9. (Epub 2021 Aug 4 PMID: 34363762)
Redman JM, Tsai YT, Weinberg BA, Donahue RN, Gandhy S, Gatti-Mays ME, Abdul Sater H, Bilusic M, Cordes LM, Steinberg SM, Marte JL, Jochems C, Kim SS, Marshall JL, McMahon S, Redmond E, Schlom J, Gulley JL, Strauss J (2022) A randomized phase II Trial of mFOLFOX6 + bevacizumab alone or with adcea vaccine + avelumab immunotherapy for untreated metastatic colorectal cancer. Oncologist 27(3):198–209. https://doi.org/10.1093/oncolo/oyab046.PMID:35274710;PMCID:PMC8914498
Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387(10027):1540–1550. https://doi.org/10.1016/S0140-6736(15)01281-7
Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G (2019) KEYNOTE-042 investigators pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393(10183):1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF (2017) KEYNOTE-045 investigators pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376(11):1015–1026. https://doi.org/10.1056/NEJMoa1613683
Powles T, Plimack ER, Soulières D, Waddell T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko I, Azevedo SJ, Borchiellini D, McDermott RS, Bedke J, Tamada S, Yin L, Chen M, Molife LR, Atkins MB, Rini BI (2020) Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 21(12):1563–1573. https://doi.org/10.1016/S1470-2045(20)30436-8
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J (2020) KEYNOTE-522 investigators pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. https://doi.org/10.1056/NEJMoa1910549
Ferrucci PF, Di Giacomo AM, Del Vecchio M, Atkinson V, Schmidt H, Schachter J, Queirolo P, Long GV, Stephens R, Svane IM, Lotem M, Abu-Amna M, Gasal E, Ghori R, Diede SJ, Croydon ES, Ribas A, Ascierto PA (2020) KEYNOTE-022 international team KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer 8(2):e001806. https://doi.org/10.1136/jitc-2020-001806
Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, Wulf G, Spring L, Sinclair NF, Andrews C, Pittenger J, Richardson ET 3rd, Dillon D, Lin NU, Overmoyer B, Partridge AH, Van Allen E, Mittendorf EA, Winer EP, Krop IE (2020) Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol 6(10):1598–1605. https://doi.org/10.1001/jamaoncol.2020.3524.PMID:32880602;PMCID:PMC7489368
Motzer R, Alekseev B, Rha SY, Porta C, Eto M, Powles T, Grünwald V, Hutson TE, Kopyltsov E, Méndez-Vidal MJ, Kozlov V, Alyasova A, Hong SH, Kapoor A, Alonso Gordoa T, Merchan JR, Winquist E, Maroto P, Goh JC, Kim M, Gurney H, Patel V, Peer A, Procopio G, Takagi T, Melichar B, Rolland F, De Giorgi U, Wong S, Bedke J, Schmidinger M, Dutcus CE, Smith AD, Dutta L, Mody K, Perini RF, Xing D, Choueiri TK (2021) CLEAR trial investigators lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384(14):1289–1300. https://doi.org/10.1056/NEJMoa2035716
Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Altan M, Jalal SI, Panwalkar A, Gubens M, Sequist LV, Saraf S, Zhao B, Piperdi B, Langer CJ (2021) Long-term overall survival from KEYNOTE-021 cohort g: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol 16(1):162–168. https://doi.org/10.1016/j.jtho.2020.09.015. (Epub 2020 Oct 15 PMID: 33069888)
Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E, Yañez E, Gümüş M, Hurtado O, de Mendoza M, Samouëlian V, Castonguay V, Arkhipov A, Toker S, Li K, Keefe SM, Monk BJ (2021) KEYNOTE-826 investigators pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N Engl J Med 385(20):1856–1867. https://doi.org/10.1056/NEJMoa2112435
Powles T, Csőszi T, Özgüroğlu M, Matsubara N, Géczi L, Cheng SY, Fradet Y, Oudard S, Vulsteke C, Morales Barrera R, Fléchon A, Gunduz S, Loriot Y, Rodriguez-Vida A, Mamtani R, Yu EY, Nam K, Imai K, Homet Moreno B, Alva A (2021) KEYNOTE-361 investigators pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): a randomised, open-label, phase 3 trial. Lancet Oncol 22(7):931–945. https://doi.org/10.1016/S1470-2045(21)00152-2
Winer EP, Lipatov O, Im SA, Goncalves A, Muñoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, Ohtani S, Turner N, Zambelli S, Harbeck N, Andre F, Dent R, Zhou X, Karantza V, Mejia J, Cortes J (2021) KEYNOTE-119 investigators pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 22(4):499–511. https://doi.org/10.1016/S1470-2045(20)30754-3
Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, Kojima T, Metges JP, Li Z, Kim SB, Cho BC, Mansoor W, Li SH, Sunpaweravong P, Maqueda MA, Goekkurt E, Hara H, Antunes L, Fountzilas C, Tsuji A, Oliden VC, Liu Q, Shah S, Bhagia P, Kato K (2021) KEYNOTE-590 investigators Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398(10302):759–771. https://doi.org/10.1016/S0140-6736(21)01234-4
Diaz LA Jr, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs P, de la Fourchardiere C, Rivera F, Elez E, Le DT, Yoshino T, Zhong WY, Fogelman D, Marinello P, Andre T (2022) KEYNOTE-177 investigators pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): final analysis of a randomised, open-label, phase 3 study. Lancet Oncol 23(5):659–670. https://doi.org/10.1016/S1470-2045(22)00197-8
Makker V, Colombo N, Casado Herráez A, Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S, Ray-Coquard I, Shapira-Frommer R, Ushijima K, Sakata J, Yonemori K, Kim YM, Guerra EM, Sanli UA, McCormack MM, Smith AD, Keefe S, Bird S, Dutta L, Orlowski RJ, Lorusso D (2022) Study 309–KEYNOTE-775 investigators lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386(5):437–448. https://doi.org/10.1056/NEJMoa2108330
Chung HC, Kang YK, Chen Z, Bai Y, Wan Ishak WZ, Shim BY, Park YL, Koo DH, Lu J, Xu J, Chon HJ, Bai LY, Zeng S, Yuan Y, Chen YY, Gu K, Zhong WY, Kuang S, Shih CS, Qin SK (2022) Pembrolizumab versus paclitaxel for previously treated advanced gastric or gastroesophageal junction cancer (KEYNOTE-063): a randomized, open-label, phase 3 trial in Asian patients. Cancer 128(5):995–1003. https://doi.org/10.1002/cncr.34019
Reckamp KL, Redman MW, Dragnev KH, Minichiello K, Villaruz LC, Faller B, Al Baghdadi T, Hines S, Everhart L, Highleyman L, Papadimitrakopoulou V, Neal JW, Waqar SN, Patel JD, Gray JE, Gandara DR, Kelly K, Herbst RS (2022) Phase II randomized study of ramucirumab and pembrolizumab versus standard of care in advanced non-small-cell lung cancer previously treated with immunotherapy-lung-map S1800A. J Clin Oncol 40(21):2295–2306. https://doi.org/10.1200/JCO.22.00912
Powles T, Yuen KC, Gillessen S, Kadel EE 3rd, Rathkopf D, Matsubara N, Drake CG, Fizazi K, Piulats JM, Wysocki PJ, Buchschacher GL Jr, Alekseev B, Mellado B, Karaszewska B, Doss JF, Rasuo G, Datye A, Mariathasan S, Williams P, Sweeney CJ (2022) Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat Med 28(1):144–153. https://doi.org/10.1038/s41591-021-01600-6
Massari F, Mollica V, Rizzo A, Cosmai L, Rizzo M, Porta C (2020) Safety evaluation of immune-based combinations in patients with advanced renal cell carcinoma: a systematic review and meta-analysis. Expert Opin Drug Saf 19(10):1329–1338
Thompson JA, Schneider BJ, Brahmer J et al (2020) NCCN guidelines insights: management of immunotherapy-related toxicities. Version. J Natl Compr Canc Netw 18(3):230–241
Kennedy LB, Salama AKS (2020) A review of cancer immunotherapy toxicity. CA Cancer J Clin 70(2):86–104
Luo J, Beattie JA, Fuentes P et al (2021) Beyond steroids: immunosuppressants in steroid-refractory/resistant immune related adverse events. J Thorac Oncol S1556–0864(21):02291–02297
Rini BI, Atkins MB, Plimack ER et al. (2021) Characterization and Management of Treatment-emergent Hepatic Toxicity in Patients with Advanced Renal Cell Carcinoma Receiving First-line Pembrolizumab plus Axitinib. Results from the KEYNOTE-426 Trial. Eur Urol Oncol S2588–9311(21): 00113–9
Albiges L, Powles T, Staehler M et al (2019) Updated European association of urology guidelines on renal cell carcinoma: immune checkpoint inhibition is the new backbone in first-line treatment of metastatic clear-cell renal cell carcinoma. Eur Urol 76(2):151–156
Rizzo A, Mollica V, Santoni M, Ricci AD, Rosellini M, Marchetti A, Montironi R, Ardizzoni A, Massari F (2022) Impact of clinicopathological features on survival in patients treated with first-line immune checkpoint inhibitors plus tyrosine kinase inhibitors for renal cell carcinoma: a meta-analysis of randomized clinical trials. Eur Urol Focus 8(2):514–521. https://doi.org/10.1016/j.euf.2021.03.001. (Epub 2021 Mar 11 PMID: 33714725)
Powles T (2021) ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 32(3): 422–423
Massari F, Rizzo A, Mollica V et al (2021) Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer 154:120–127
Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U (2019) ESMO guidelines committee electronic address: clinicalguidelines@esmo.org. Cutaneous melanoma: ESMO clinical practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol 30(12):1884–1901. https://doi.org/10.1093/annonc/mdz411
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
A.R. wrote the main manuscript text and performed the statistical analysis. A.R. prepared figures 1-5 and table 1. V.M., V.T., E.T., A.M., and M.R. contributed to data acquisition. V.M., R.D.L., M.S., and F.M. contributed to the conception, design, and manuscript revision. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. No writing assistance was utilized in the production of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rizzo, A., Mollica, V., Tateo, V. et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother 72, 1381–1394 (2023). https://doi.org/10.1007/s00262-023-03366-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-023-03366-x